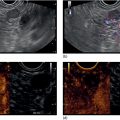
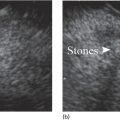
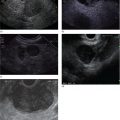
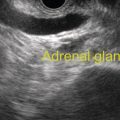
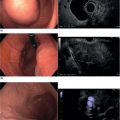
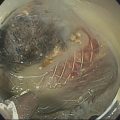
Sam M. Serouya1 and Adam J. Goodman2 1 NYU Langone Grossman School of Medicine, New York, NY, USA 2 NYU Langone Health, New York, NY, USA Pain that develops as a result of pancreatic cancer (Figure 35.1) or chronic pancreatitis (Figure 35.2) is often difficult to manage. Up to 90% of patients with advanced pancreatic cancer report pain that significantly impacts their quality of life. Various methods have been used to treat these patients including narcotic analgesia, antidepressants, pancreatic enzymes, octreotide, denervation procedures, and palliative or decompression/drainage procedures. Opioid analgesics can sometimes effectively treat pain, but are often inadequate and can be associated with numerous side effects including constipation, delirium, nausea, and the potential for addiction. Non‐pharmacologic methods of pain control, such as celiac plexus block or neurolysis, may improve quality of life and minimize drug‐related side effects. The celiac plexus lies anterior to the aorta at the level of the celiac artery (Figure 35.3). Most of the sensory nerves returning from the pancreas and other intra‐abdominal viscera pass through the celiac ganglion and splanchnic nerves and interruption of these fibers may lessen pain in pancreatic malignancies and chronic pancreatitis. Celiac plexus block (CPB) is a temporizing treatment where a steroid and long‐acting local anesthetic are injected into the celiac plexus to control pain associated with chronic pancreatitis. Celiac plexus neurolysis (CPN) is used in patients with pancreatic cancer and refers to the injection of alcohol or phenol, a more permanent agent, into the celiac plexus in an attempt to ablate the nerve fibers that transmit pain. The history of CPN dates back to 1914 when Kappis and colleagues described an intraoperative procedure. Many decades later, in 1996, the first endoscopic ultrasound (EUS)‐guided CPB/CPN was described by both Faigel and colleagues, and Wiersema and colleagues. Then, in 1999, Gress and colleagues described the first case of EUS‐guided CPB for chronic pancreatitis. The efficacy of CPN for the treatment of cancer pain has been demonstrated in a number of studies, with pain control in 70–90% of patients up to three months after the procedure. CPB may have a role in the treatment of pain related to chronic pancreatitis. One study consisting of 18 patients with chronic pancreatitis noted a reduction in pain in 50% (5/10) of patients undergoing EUS‐guided CPB compared with 25% (2/8) of patients undergoing computed tomography (CT)‐guided blocks. The benefit persisted for up to 24 weeks in 30% of responders. Other studies have demonstrated significant improvement in overall pain scores in approximately 55% of patients at up to eight weeks of follow‐up; however, only 10% of patients noted a persistent benefit beyond 24 weeks. These studies have also demonstrated that response to the first EUS‐CPB is associated with response to subsequent blocks, thus suggesting repeat procedures will be of benefit to the patient. Patients with inoperable pancreatic cancer and pain requiring narcotic analgesics are potential candidates for CPN. Selection of patients with pain related to chronic pancreatitis for CPB is less clear; however, patients wishing to avoid narcotics or those with pain refractory to high doses of narcotics may be appropriate candidates. Patients should be selected carefully, with contraindications including platelets less than 50 × 109/l, coagulopathy, disulfiram use, and prior bowel obstruction secondary to CPB/CPN. Prior to the procedure the patient is hydrated with intravenous saline (500–1000 ml), in order to prevent postprocedural hypotension. The patient is then placed in the left lateral decubitus position and sedation is administered. The linear array echoendoscope is advanced to just below the gastroesophageal junction where a sagittal view of the aorta is obtained through the posterior gastric wall. The aorta is traced to the celiac trunk, the first large vessel emerging off the aorta, and the second branch is usually easily identified as the superior mesenteric artery (Figure 35.4). Identifying both the celiac and superior mesenteric arteries helps to confirm correct location. Color flow Doppler should be used to exclude any intervening vessels. The stylet is removed from a fine needle aspiration (FNA) needle and the entire system is flushed with normal saline to remove any air as this can interfere with imaging. Using real‐time imaging, the FNA needle is inserted immediately lateral and anterior to the aorta at the level of the celiac trunk via a transgastric approach (Figure 35.5). A small amount (2 ml) of 0.9% saline is injected to clear the needle. An assistant then aspirates a 10‐ml syringe filled with 0.9% saline for approximately 10 seconds. This is to confirm that the needle is not within any of the regional blood vessels. Injections into the celiac axis can be performed in two ways: one in which the injection is performed with the entire amount of medication infused at a single site at the base of the celiac artery take‐off, and one in which injections are performed by rotating to each side of the celiac trunk. For CPB, 20 ml of preservative‐free bupivacaine (0.25%) followed by 80 mg of triamcinolone is injected, followed by 3–5 ml of saline. For CPN, 20 ml of bupivacaine (0.25%) followed by 20 ml of dehydrated alcohol is injected at the base of the celiac artery. After alcohol injection, the needle is flushed with 3 ml of saline prior to withdrawal. Figure 35.1 Hypoechoic pancreatic mass as seen by EUS. Figure 35.2 Endoscopic ultrasound demonstration of lobularity, one of the major criteria to help diagnose chronic pancreatitis by EUS. Figure 35.3 Celiac axis (CA) as seen by EUS. AO, aorta. Figure 35.4 Celiac axis confirmed by the aorta, celiac artery (CA) and superior mesenteric artery (SMA). Figure 35.5 Insertion of the EUS‐guided fine needle aspiration needle into the area of the celiac axis. Alternatively, two separate injections are performed by rotating to each side of the celiac trunk. With this technique, 10 ml of bupivacaine (0.25%) and 10 ml of dehydrated alcohol in CPN and 10 ml of bupivacaine (0.25%) followed by 40 mg (1 ml) of triamcinolone in CPB are injected on each side of the celiac trunk. The aspiration test is repeated before each injection. It is believed that the two techniques have similar results. The examination usually takes 30 minutes and is performed on an outpatient basis. Recovery time is usually two hours. Before discharge, orthostatic blood pressure changes should be checked, and patients should be counseled regarding potential complications. Endoscopic ultrasound‐guided CPB and CPN are generally effective, safe, and well‐tolerated procedures. The three most common complications are transient hypotension (20–40%) due to dilation of the splanchnic vasculature, transient diarrhea (4–38%) due to blockade of the sympathetic innervation of the abdominal viscera and unopposed parasympathetic activity, and transient increase in pain (9%). These symptoms are usually limited to two days post procedure. Less common complications include unilateral paresis or paraplegia, pneumothorax, loss of sphincter function, retroperitoneal bleeding, renal puncture, and prolonged gastroparesis. In addition, cephalic spread of the neurolytic agent may result in involvement of the cardiac nerves and plexus. Infectious complications are uncommon but potentially serious. In a series of 90 patients, only one patient developed an infectious complication (a peripancreatic abscess), which resolved with a two‐week course of antibiotics.
35
How to do Celiac Plexus Block
Introduction
Technique (Video 35.1)





Complications
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree