Abstract
Hypoplastic left heart syndrome (HLHS) is one of the most common prenatally diagnosed congenital heart diseases because it usually profoundly alters the four-chamber view. There is a high risk of associated chromosomal, cardiac, and extracardiac defects. HLHS is associated with poor survival rates and quality of life in survivors.
Keywords
heart defect, left ventricle tract obstruction, hypoplastic left heart syndrome
Introduction
Hypoplastic left heart syndrome (HLHS) occurs in 0.1 : 1000 to 0.6 : 1000 live births and accounts for 2% to 3% of all congenital heart diseases (CHDs). HLHS is one of the most severe CHDs, with a high rate of perinatal death. Without treatment, it is responsible for 25% to 40% of all neonatal cardiac deaths.
Disease
Definition
HLHS encompasses a spectrum of congenital heart malformations characterized by underdevelopment of the left heart structures.
Prevalence and Epidemiology
HLHS represents 2% to 3% of all CHDs. Because it is usually easily diagnosed on a four-chamber view, widespread introduction of ultrasound (US) screening significantly improved the detection rate. HLHS is associated with a poor prognosis and an intrauterine mortality rate of about 5%. Males are more frequently affected than females. There is a 2% to 4% recurrence risk. Autosomal recessive inheritance has occasionally been described.
Etiology and Pathophysiology
The etiology is multifactorial. HLHS has been described as part of multiple syndromes. Mutations of NKX2 and NOTCH1 genes and proteins 43 and 11q23.3 have been identified. Chromosomal anomalies have been described in 5% to 12% of cases, especially in cases with other cardiac defects, mainly atrioventricular septal defects and aortic coarctation. Commonly found chromosomal anomalies include monosomy X and trisomies 18 and 13. Extracardiac anomalies, mainly central nervous system defects, are present in 15% to 30% of cases.
HLHS is associated with hypoplasia and lack of function of the left heart. The right ventricle supports the systemic circulation via the ductus arteriosus. Several forms of HLHS have been reported.
- •
The most frequent form includes mitral and aortic atresia. The lack of flow across left heart structures results in underdevelopment of the left atrium and ventricle, the mitral and aortic valves, and the aortic arch ( Fig. 81.1 ).

Fig. 81.1
(A) Slitlike left ventricle (asterisk) secondary to mitral and aortic atresia. There is no left cavity growth. The septum primum helps differentiate left from right structures (dashed arrow) . (B) Color Doppler evaluation shows flow across the tricuspid valve, whereas the mitral valve permits no flow across it.
- •
A second, relatively common form is characterized by critical aortic stenosis and aortic atresia with a hypoplastic but not atretic mitral valve. In contrast to cases with mitral atresia, the left ventricle is globular and hypertrophic and eventually evolves to left ventricular hypoplasia ( Fig. 81.2 , ).

Fig. 81.2
Hypoplastic left ventricle secondary to aortic atresia. Left ventricle cavity is reduced (LV) ; however, the left ventricle walls are hypertrophic, simulating a myocardial tumor.
- •
More rarely, HLHS can be the end stage of severe forms of other cardiac defects, including the following:
- •
Mitral atresia with double-outlet right ventricle, which includes mitral atresia and a perimembranous ventricular septal defect. The aortic valve is usually not atretic, and the aorta shows antegrade flow across the aortic arch. The outflow tracts can be either properly arranged or transposed ( Fig. 81.3 ).

Fig. 81.3
Mitral atresia with double-outlet right ventricle, transposition of great arteries, and pulmonary stenosis. The mitral valve (arrow) is closed and permits no flow across it (A). There are two outflow tracts (B) with antegrade flow (C). The aorta (Ao) is anterior and to the right of the pulmonary artery (p), which is smaller than the aorta (B).
- •
Unbalanced atrioventricular septal defects with marked asymmetry between ventricles and great arteries. When the aorta and left ventricle are hypoplastic, univentricular correction may be required.
- •
Severe forms of aortic coarctation and interruption of the aortic arch.
- •
Manifestations of Disease
Clinical Presentation
HLHS is usually asymptomatic prenatally. Infants are generally born at full term and appear healthy initially. Because HLHS is a duct-dependent anomaly, prostaglandin administration is required at birth. With closure of the ductus arteriosus, systemic perfusion becomes abruptly decreased, resulting in hypoxemia, acidosis, and cardiogenic shock.
Imaging Technique and Findings
Ultrasound.
HLHS is one of the most commonly detected forms of fetal heart disease. Left heart structures are underdeveloped, which typically alters the four-chamber view:
- •
The four-chamber view shows a hypoplastic left atrium and ventricle in cases with mitral and aortic atresia. There is no flow across the atrioventricular or the semilunar valve, with a resultant slitlike left ventricle. Color Doppler shows no flow across the mitral valve (see Fig. 81.1A and B ). In contrast, when the mitral valve is patent and there is aortic atresia, the left ventricle is hypertrophic and echogenic with endocardial fibroelastosis. Color Doppler may show mitral regurgitation (see Fig. 81.2 , ).
- •
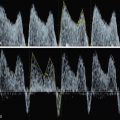
The most severe cases show reversed flow in the pulmonary veins ( Fig. 81.4 ) secondary to pulmonary hypertension and arterialization and high left atrial pressure.