David J. Breen, Elizabeth E. Rutherford, Beth Shepherd
Image-Guided Biopsy and Ablation Techniques
Image-Guided Biopsy
Introduction
There is an increasing role for imaging in the planning and performing of biopsy procedures. It is becoming unacceptable in modern practice to perform ‘blind’ liver or renal biopsies when imaging can provide real-time monitoring of needle position and hence reduce complication rates.1,2 Ongoing technological advances are providing us with new and improved imaging modalities to aid biopsy and there are a number of modality fusion techniques which promise to further improve lesion targeting in the future.
Principles of Image-Guided Biopsy
Percutaneous image-guided needle biopsy is now a standard technique for the diagnosis of most tumours throughout the body and also has a role in the diagnosis of certain infective and inflammatory conditions. It is particularly helpful in the staging of cancer, most notably when the definitive treatment may not involve surgical intervention. Advances in imaging techniques have led to greater precision in the targeting of lesions. Advantages of percutaneous biopsy over surgical excision biopsy include time and cost savings and reduced morbidity. The complication rates associated with percutaneous biopsy vary according to the organ studied, but are generally lower than 0.1%.3
Case Selection
Most image-guided biopsies can be performed using local anaesthesia and sedation. General anaesthesia may be preferable in some patients, including children. Pertinent patient history includes bleeding diatheses and anti-coagulant usage. Contraindications to biopsy include an uncorrected coagulopathy and lack of a safe needle approach route. The benefits of confirming a suspected diagnosis need to be evaluated against the inherent procedural morbidity.
Pre-Procedural Assessment
Pre-procedural assessment aims to reduce complication rates by optimising the patient’s physiology and identifying contraindications in a timely fashion. It is also useful in alleviating patient anxiety and discussing post-procedural care to enable the patient to plan time off work, etc. The pre-procedural assessment is also a good time to obtain patients’ written consent.
Careful questioning can determine whether a day case procedure is appropriate, taking into account the patient’s clinical status and home circumstances. Any language, cultural or religious barriers can also be identified. Regularly updated and referenced departmental patient information leaflets should be available for all common procedures and include links to web-based information and relevant telephone numbers for further advice. These help inform the consent process and provide post-procedural advice as it is well documented that patients retain little of any verbal information they are given.4
Another role of pre-procedural assessment is to highlight potential problems related to patient co-morbidity or the nature of the biopsy target. If the patient is on anticoagulants or has a coagulopathy, the timing of the biopsy procedure should be carefully planned around the cessation of anticoagulant medication or admission for correction of coagulation disorders. These patients have an increased risk of post-procedural haemorrhage and may require an extended period of observation. The nature of the target lesion also needs consideration as vascular lesions are at increased risk of bleeding; it may be necessary to establish peripheral venous access and ensure the availability of cross-matched blood. Wherever possible, an obstructed organ should be decompressed prior to biopsy using techniques such as biliary drainage or percutaneous nephrostomy.
Patients should be told how and when they will receive biopsy results and a follow-up outpatient clinic appointment booked prior to discharge.
Core Biopsy vs Fine Needle Aspiration
Fine needle aspiration (FNA) utilises a small calibre needle (20 to 25G) to obtain a sample of cells from a target organ/lesion for cytological analysis. Core biopsy involves larger calibre needles (14 to 19G) and reveals more structural information, which is often necessary for histological diagnosis (Table 87-1).5,6 Each method has its own advantages and disadvantages and the decision as to which one to use depends on many factors:
TABLE 87-1
Needle Gauge and Calibre
| Needle Gauge | Diameter (mm) |
| 22 | 0.72 |
| 21 | 0.82 |
| 19 | 1.10 |
| 18 | 1.26 |
| 16 | 1.67 |
| 14 | 2.13 |
FNA with a small calibre needle may be employed in situations where a structure (e.g. bowel loop) needs to be transgressed as it is interposed between the target lesion and skin. Similarly, where a deep lesion lies in close proximity to critical vascular structures (e.g. a central liver lesion abutting the vena cava), core biopsy may be deemed too hazardous.
Aside from the reduced risk of iatrogenic injury, FNA can be useful in frail or unwell patients who cannot tolerate a prolonged procedure. A further advantage is that cytological slides can be examined immediately to check for adequacy and formal pathology reports can be issued rapidly if required.
The degree of confidence in cytological diagnosis will vary according to the indication; the diagnosis of recurrent malignancy can be more easily made on a cytological sample if previous tumour tissue is available for comparison. For a new diagnosis of malignancy, however, a larger sample of tissue is usually required and hence core biopsy is generally preferred over cytology. This is particularly important where different subtypes of malignancy exist (e.g. lymphoma), and accurate histological typing is necessary to plan treatment.7–9 Other pathologies requiring histological confirmation include diffuse disease such as cirrhosis and renal parenchymal disease (e.g. glomerulonephritis).
Biopsy Needles
Needles vary in calibre, tip design, length and mechanism of action and there are a wide range of different products on the market. Needle choice depends on a number of factors including the lesion to be targeted, the number of cores required, the modality chosen for image guidance, personal preference, cost and local availability. They can be broadly classified as follows.
Fine Needle Aspiration Cytology Needles
Cytological samples are usually taken with small calibre needles (20–25G). Different lengths of small calibre needle are available and the needle should be carefully selected according to the depth and size of lesion to be sampled. For abdominal FNA, the target will often be deep and a spinal or other stylet needle is often used. The presence of a needle stylet helps to avoid luminal contamination with tissue before it reaches its target. When using very fine needles, a stylet also aids insertion by stiffening the needle to prevent it deviating from its course.
Core Biopsy Needles
For core biopsy, larger calibre cutting needles are employed (usually 16–18G for abdominal biopsies). The size of biopsy needle chosen depends on the organ being targeted and may also vary according to the number of samples being obtained. For a routine ‘background’ liver biopsy, a single pass is usually sufficient and so a larger (e.g. 16G) biopsy needle may be chosen. If the operator anticipates that several cores will need to be obtained, e.g. in the case of multiple lesions, then an 18G needle may be more appropriate to reduce the risk of bleeding following multiple liver capsule punctures.
The shape of the tissue core obtained varies according to the design of the chamber within the biopsy needle; various manufacturers have designed biopsy instruments which optimise the volume of tissue obtained for a given needle gauge10 as there is evidence that increasing needle calibre is related to increased risk of haemorrhage.11
Menghini Technique Biopsy Needles, e.g. Surecut
These operate using a suction mechanism but are no longer in common use. They are based on the Menghini principle in which the needle, stylet and syringe form a single unit.
Sheathed Biopsy Needles
Manual, e.g. Tru-Cut
These needles consist of a biopsy chamber that can be opened and closed. After insertion to the correct depth, the needle is opened by manually advancing the inner portion so that the surrounding tissue falls into the biopsy notch. As the outer part of the needle is then advanced, the tissue in the chamber is cut by its leading edge. This requires two hands to operate and so is impractical for ultrasound-guided procedures.
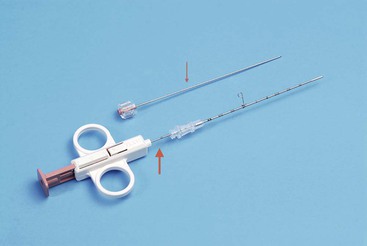
Semi-Sutomatic, e.g. Temno, SuperCore (Fig. 87-1)
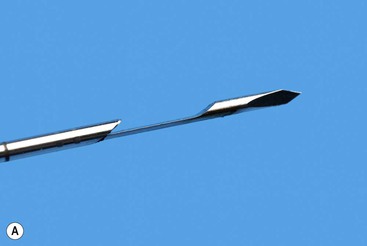
Some biopsy instruments allow placement of the central notch at the exact position required and then fire the cutting sheath over the stylet (Fig. 87-2). There is therefore no additional forward excursion of the needle upon firing the cutting part of the needle, so minimising the risk of damage to adjacent structures. This is useful in the case of small lesions or those adjacent to critical structures such as large veins. When the target tissue is fibrous or very firm in texture it can, however, be difficult to manually advance the cutting needle through the lesion without displacing it. This is often overcome by the use of spring-loaded fully automated devices that exert more forward force to advance the needle into the target.
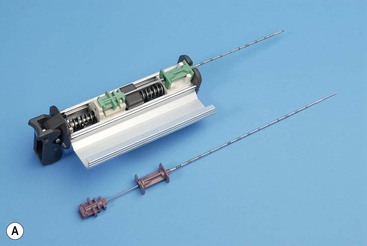
Fully Automatic, e.g. Biopty gun, Achieve, Biopince, Bard Max-Core (Fig. 87-3)
These can take the form of metal biopsy guns, which are designed for use with disposable biopsy needles of different gauges, or fully disposable integrated plastic biopsy devices, which have a similar mechanism of action. Whilst the disposable devices are more expensive, they avoid the difficulties associated with sterilisation of the metal biopsy guns and have generally been adopted as the biopsy instrument of choice in many radiology departments. Fully automatic biopsy instruments fire both a central stylet and cutting sheath in a rapid forward motion such that the tissue core is obtained at a preset distance or ‘throw’ (e.g. 2 cm) ahead of the visualised needle tip. Many of the instruments offer a choice of throw (usually 1 or 2 cm) depending on the size of target lesion or organ. This mechanism of action means that the operator needs to be aware of the size of the lesion to be sampled relative to the throw of the biopsy needle. Often the needle tip can be positioned at the superficial margin of the target lesion to avoid injury to adjacent structures.
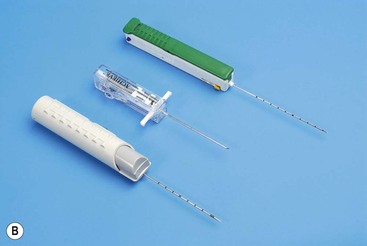
Coaxial Technique
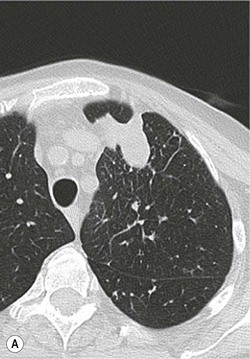
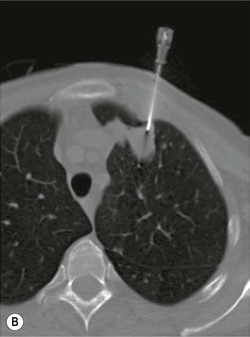
Most biopsies are performed by making one or more passes into an organ or mass with a single biopsy needle. Occasionally it may be helpful to employ a system whereby a coaxial needle is radiologically guided into the lesion/mass, the central stylet is removed and then a smaller calibre biopsy needle can be inserted through the coaxial system to obtain multiple cores (Fig. 87-4). This has the advantage of allowing several samples to be taken without re-puncturing the superficial soft tissues or organ capsule, which theoretically reduces the risk of haemorrhage and time taken to target the lesion,12 although studies have not demonstrated any significant difference in complication rates between coaxial and non-coaxial techniques.13 The coaxial needle can be angled between samples to increase the volume of tissue sampled. This technique is commonly employed during CT-guided biopsy, particularly for lung lesions where minimising the number of passes through the pleura, reduces the risk of pneumothorax (Fig. 87-5). It is also used for biopsy of deep abdominal/pelvic masses, as it reduces the radiation dose and trauma involved in re-positioning biopsy needles for each core. There is also a theoretical advantage that coaxial systems reduce the risk of track seeding. A disadvantage is that the calibre of the coaxial needle must be larger than that of the biopsy needle, increasing the overall size of the needle track, with a possible increased risk of damage to adjacent tissues.
Imaging Modalities for Biopsy
Choice of modality for biopsy varies according to the size, location and visibility of the lesion. Most intra-abdominal lesions can be approached using either ultrasound or CT imaging. Modern technology is increasingly allowing a combination of modalities to be used during biopsy procedures (fusion imaging) to aid targeting of lesions. Previously, this required time-consuming manual registration of imaging but newer automated registration/fusion software is considerably reducing the time taken for co-registration of different modalities and it is likely that this technique will become commonplace in the future.
Ultrasound
Ultrasound-guided biopsy is considered an accurate, safe, widely accessible and relatively cheap technique. It has the advantage of real-time visualisation of the needle (particularly important for biopsy of organs such as the liver where the time the biopsy needle remains within liver parenchyma should be limited to minimise haemorrhagic complications). In addition, it allows a multiplanar angled approach that may be more challenging at CT or MR imaging. Ultrasound-guided biopsy procedures have the advantages of portability and lack of ionising radiation. However, ultrasound-guided biopsies are more operator-dependent than procedures guided by other imaging modalities. Ultrasound platforms and software are becoming increasingly advanced, enabling the operator to benefit from additional information to aid lesion targeting. Intravenous ultrasound contrast agents and elastography data are being used with increasing frequency.
Technology which enables co-registration of real-time ultrasound imaging with CT/MRI studies is becoming more accessible.14 One example of this is using MRI data to aid ultrasound-guided prostate biopsy. It can be difficult to identify focal prostatic lesions at ultrasound and cancers may be missed despite methods which allow a large number of cores to be acquired. Fusing pre-procedural MRI data with real-time transrectal ultrasound (TRUS) imaging combines the advantages of each modality by allowing the biopsy needle to be introduced into suspicious lesions previously identified with MRI but under real-time TRUS guidance.15
CT
CT is generally preferred for biopsies of the lung, bone and spine. CT is also particularly useful for biopsies of deep abdominal lesions where ultrasound visualisation is poor, for example retroperitoneal nodal masses. CT-guided techniques can be extremely accurate with appropriate lesion selection and an experienced user. However, access to scanning time and radiation dose are limiting factors. Additionally non-fluoroscopic CT techniques are not ‘real time’, as needle position is checked after each adjustment. This theoretically carries a greater risk of damaging local structures compared to true real-time techniques. CT fluoroscopy technology enables biopsies to be performed under real-time CT imaging. This is useful for more challenging procedures (e.g. small target lesions) and may be performed with relatively small radiation doses.16 CT fusion techniques, for instance with ultrasound, are increasingly used as discussed elsewhere.
MRI
Recent advances in MRI have made it increasingly useful, particularly where an oblique approach is required, e.g. for subdiaphragmatic liver and adrenal masses.17,18 MRI also provides improved soft tissue detail when imaging certain organs and structures compared with CT or ultrasound and hence can be invaluable in targeting lesions in areas such as the prostate which are difficult to visualise with ultrasound or certain liver lesions which cannot be appreciated on unenhanced CT imaging.19,20
MRI-guided breast biopsies are also performed, particularly for lesions that are difficult to localise with mammography or ultrasound. Within the field of musculoskeletal imaging, MRI-guided biopsy is useful for lesions in the bone marrow that cannot be identified with CT. The use of MRI guidance is likely to increase as the technology improves.
The main disadvantage of MRI-guided biopsies is the magnetic environment, which limits equipment usage, making some procedures more complex. This in turn can lead to increased procedural times which may be an issue where MRI capacity is limited. There are also increased costs to consider, and patients may find MRI-guided procedures more uncomfortable due to positioning constraints. Open magnets allow direct access to the patient during the entire procedure, enabling real-time monitoring of needle insertion. A further problem with MRI-guided biopsy is the need for motion correction during the biopsy procedure. In addition, patients may not be able to tolerate MRI due to claustrophobia or have other contraindications such as pacemakers. MRI-safe biopsy equipment is now mass-produced but is expensive and the range is limited.
PET CT
The mechanism by which PET images are obtained is an intrinsic barrier to their use in biopsy, in that images are obtained over a period of time and proximity to the patient during acquisition results in radiation safety issues. However, novel fusion techniques that combine real-time CT with pre-procedural PET/CT to identify lesions that are not easily visualised on conventional CT and are only identified due to their differential FDG uptake have been described.21
Fluoroscopy
Plain fluoroscopic biopsy techniques are most frequently used for musculoskeletal lesions, as bony landmarks are easily identified. Fusion techniques are being increasingly developed, for example the use of pre-procedural MRI and CT overlaid on real-time fluoroscopic images; this is useful for sampling multiple targets, including head and neck lesions.22
Fluoroscopy is also utilised in transvascular biopsy techniques, where intravascular contrast is used to guide access, e.g. transvenous liver biopsy via the jugular or femoral vein,23 which is usually performed when liver biopsy is essential but contraindicated percutaneously.
Stereotactic
This technique is mainly limited to the biopsy of breast lesions that are seen on mammography but cannot be visualised with ultrasound. They are performed most commonly for microcalcification and architectural distortion. This technique relies on computer software to interpret angled mammography views to give a three-dimensional location of the lesion.
Endoscopy/Endoscopic Ultrasound (EUS) and Bronchoscopy/Bronchoscopic Ultrasound
More often performed by appropriately trained clinicians than radiologists, direct visualisation biopsies and endoscopic/bronchoscopic ultrasound-guided aspiration or biopsy procedures have an important role in obtaining tissue from areas which are difficult to access percutaneously. EUS can also be combined with elastography to aid lesion identification and biopsy.24
Tips and Tricks
‘Look Before you Leap’—Procedural Set Up
As with any procedure, time spent reviewing pre-procedural imaging and planning biopsy approach and modality is very well spent. Particularly when performing ultrasound-guided procedures, the operator should try and ensure that the patient’s bed is at an appropriate height. Small changes to patient position and room setup can increase or decrease significantly the difficulty of performing the biopsy.
Avoiding Inadequate Samples
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree