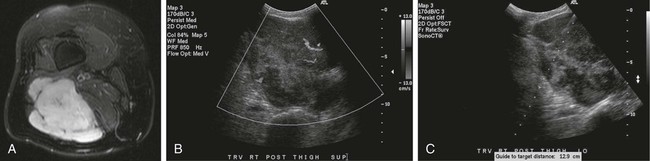
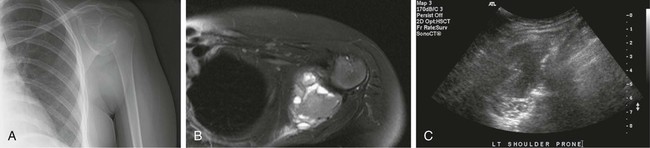
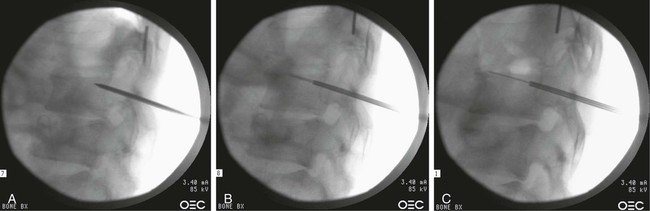
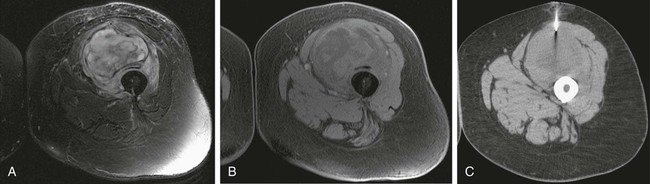
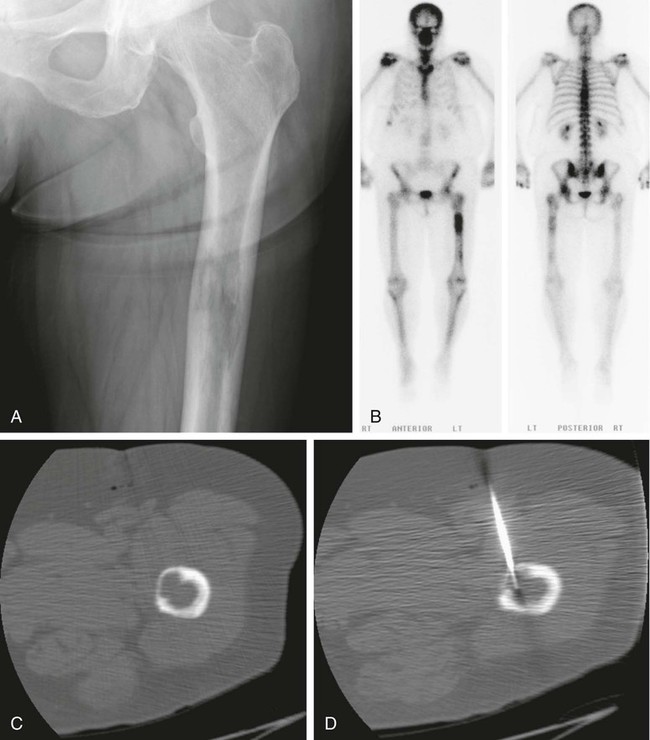
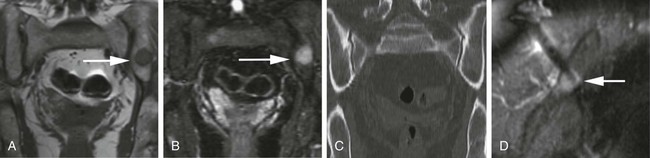
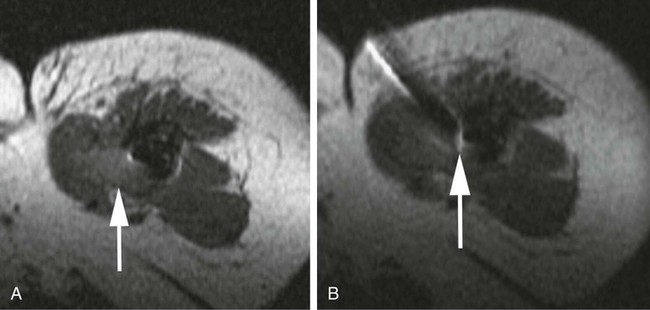
Image-guided percutaneous needle biopsy (PNB) has emerged as a safe, effective, and accurate tool for the diagnosis of musculoskeletal lesions.1–5 Information obtained from percutaneous biopsy helps determine appropriate therapy and disease prognosis. Whether a lesion is benign or malignant and its specific histologic type and grade (in the case of malignant lesions) is vital knowledge for treatment planning. Because diagnostic accuracy may be greatly influenced by the biopsy method used and location of sampling, a well-planned and well-executed percutaneous biopsy is essential for providing an accurate diagnosis and facilitating treatment. When a biopsy is performed poorly, the outcome may be disastrous from a number of standpoints. If an incorrect diagnosis is obtained, a delay in treatment may result, and complications may ensue. In addition, when the percutaneous route taken is badly planned, treatment options can become limited, thus endangering potential limb preservation surgery and creating a significant negative impact on ultimate survival.6–10 Traditionally, open incisional biopsy was considered the gold standard for ensuring that adequate tissue was obtained from a musculoskeletal lesion for proper diagnosis. In conjunction with clinical and imaging characteristics, the final histologic diagnosis would then be made. However, open biopsies have a complication rate of 16%, and 8.2% of all patients who have undergone biopsy have their treatment plan affected by these complications.8 In addition, 1.2% of patients who have had a biopsy undergo unnecessary amputations because of diagnostic errors that are based on the results of an open biopsy.8 Historically it was believed that needle biopsy was ineffective for the diagnosis of some lesions, specifically primary mesenchymal musculoskeletal tumors, because such tumors are among the most difficult of pathologies to accurately diagnose. As such, in a survey of practices in the early 1980s, only 9% of patients reportedly underwent needle biopsy,11 whereas approximately 40% of patients underwent needle biopsy in the late 1990s.12 Today, PNB is thought to be the initial procedure of choice for establishing the diagnosis of a musculoskeletal lesion.12,13 Well-established, consistently good results for core needle biopsy (CNB) have been reported.14–19 Welker et al. found no significant deleterious effects on patient outcome when diagnostic errors occurred after needle biopsy.12 Needle track seeding has not been a relevant clinical issue, since en bloc resection of the biopsy track and local field radiation is commonly practiced. The reported accuracy of a needle biopsy procedure in distinguishing benign from malignant lesions, the exact grade, and the exact pathology was 92.4%, 88.6%, and 72.7%, respectively, with a major diagnostic error rate of 1.1%, none of which ultimately had an impact on patient outcome.12 More recently, PNB was established as a highly accurate and clinically useful technique for characterizing musculoskeletal lesions, with often higher accuracy in soft-tissue masses than with bone lesions. Certain histologies such as lymphoma and histiocytosis may require a second biopsy for final diagnosis.19 In addition to having good diagnostic accuracy, additional benefits of PNB include a three- to fivefold increase in cost-effectiveness over traditional open biopsy,20–22 minimal limitation of activity after the procedure, rapid recovery time, quicker initiation of patient treatment, and assistance with operative planning.7–9,16,23 Contrary to open biopsy, PNB does not significantly alter the strength of weight-bearing areas, thus avoiding the need for immobilization.6,9,24 • Establish whether a musculoskeletal lesion is benign or malignant • Obtain material for microbiologic analysis in patients with known or suspected infection • Stage patients with known or suspected malignancy when local spread or distant metastasis is suspected • Determine the nature and extent of systemic diseases (e.g., connective tissue diseases) Percutaneous needle biopsy may be performed under fluoroscopic,25–27 ultrasound,25–28 computed tomography (CT),3,25–26 or magnetic resonance imaging (MRI) guidance.29–31 The imaging choice is primarily dependent on the location and imaging characteristics of a lesion and, to a lesser degree, on the available imaging modalities and patient positioning. For a superficial soft-tissue lesion or a lesion with cortical disruption that allows an acoustic window for passage of a needle, ultrasound may be used (Figs. 157-1 and 157-2). Ultrasound provides real-time visualization, minimal patient and preprocedure preparation, and no radiation exposure to the patient. Obviously, poor sound penetration or a hard surface precludes the use of ultrasound for deep medullary bone lesions. Moreover, deep soft-tissue lesions also preclude ultrasound use because poor sound penetration may lead to unknowing disruption of critical soft-tissue compartments. For a radiographically identifiable lesion that does not require careful negotiation of neurovascular structures, fluoroscopy allows fast multidirectional real-time visualization with ease of use and less cost than CT or MRI (Fig. 157-3). However, low soft-tissue contrast makes fluoroscopy less ideal for lesions with large cystic or necrotic areas. For a deep lesion or a lesion adjacent to neurovascular bundles, CT and MRI are ideal for creating high-resolution road maps of compartmental anatomy. In practice, CT is routinely used for lytic or blastic lesions with or without a soft-tissue component, whereas MRI is often used for lesions that are not identified on other imaging modalities and for targeting a focal marrow abnormality (Figs. 157-4 and 157-5). However, conventional CT can occasionally be a slow, arduous modality because of the lack of real-time visualization and the requirement for repeated scanning during needle manipulation and positioning. CT-fluoroscopy allows the combined convenience of real-time guidance and instantaneous cross-sectional anatomy. Although MRI guidance requires MRI-compatible (high nickel content) needles (E-Z-EM Inc., Westbury, N.Y.; Somatex, Berlin, Germany; MD Tech, Gainesville, Fla.; InVivo, Berlin, Germany), imaging with frequency encoding parallel to the needle path reduces susceptibility artifact, and specialized monitor devices and platforms are being developed for MRI-guided procedures32,33 (Figs. 157-6 and 157-7).
Image-Guided Percutaneous Biopsy of Musculoskeletal Lesions
Indications
Equipment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine