Abdominal vascular conditions demand special attention in diagnosis. First, despite highly variable presentations, they must be suspected by the clinician so that appropriate imaging can be performed. Routine abdominal imaging without vascular contrast agents can be normal in the presence of these life-threatening processes. Second, diagnostic imaging must be performed without delay, because these conditions are time sensitive and often fatal. Unfortunately, in many cases, the optimal test cannot be performed because of the patient’s hemodynamic condition, renal insufficiency, or contrast allergy. Even when the best available tests are performed, the sensitivity of diagnostic imaging for some key conditions is not perfect, so the emergency physician must remain vigilant for the possibility of a false-negative test result. We begin by defining the disease conditions and then briefly discuss their epidemiology and presentation. Although no formal clinical decision rules exist for these conditions, some risk stratification strategies can improve patient selection for diagnostic imaging. We also discuss the sensitivity and specificity of available imaging tests and interpretation of imaging findings.
Abdominal Vascular Catastrophes
Abdominal vascular catastrophes constitute a family of disorders sharing some key features but differing in details. Overall, these conditions primarily affect older patients, although rarely young patients with no apparent medical problems may be afflicted. Therefore vascular causes should always be considered in patients presenting with acute abdominal pain. According to Centers for Disease Control data, abdominal vascular diseases become an important cause of death by around age 50, with aortic aneurysm and dissection accounting for more than 13,000 U.S. deaths in 2006. Mesenteric ischemia may be more common than epidemiologic data suggest, because it may be misdiagnosed and may be a cause of death in elderly patients not undergoing premortem definitive diagnostic testing or autopsy. Here, we define each of these disease states, pointing out risk factors as well as some common misconceptions about the presentation that may prevent timely recognition.
General Approach to the Patient With Potential Abdominal Vascular Catastrophe
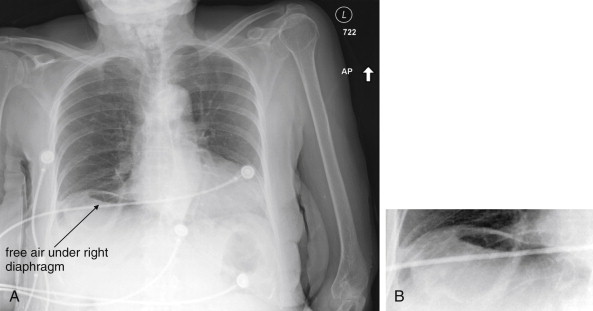
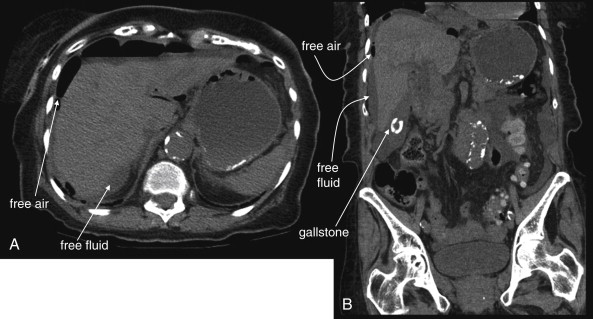
The clinical presentations of abdominal aortic aneurysm (AAA) rupture, abdominal aortic dissection, and mesenteric ischemia can overlap substantially, and a unified imaging approach to evaluate for all is best when any of the three is suspected. General resuscitation measures, preoperative preparation, and surgical consultation should occur simultaneously with imaging. Because perforation of a viscus with pneumoperitoneum can present with sudden abdominal pain simulating vascular catastrophes, an upright portable chest x-ray is a reasonable bedside screening examination ( Figure 11-1 , and CT for comparison Figure 11-2 ), although it provides no specific information about abdominal vascular disorders. Other plain radiographs should be minimized, because these provide little diagnostic information and can waste precious time. Rarely, frank pneumatosis intestinalis may be seen on plain radiographs ( Figure 11-3 ), but more often x-rays may provide false reassurance or misdiagnosis of a more benign abdominal condition. Bedside imaging with ultrasound by the emergency physician to assess for AAA should occur at the time of initial physical examination ( Figures 11-4 to 11-11 ). If AAA is not recognized by ultrasound, rapid computed tomography (CT) without oral contrast should be obtained in the stable patient. Intravenous (IV) contrast should be used when possible, as explained later. The presentation of vascular catastrophes can also overlap considerably with other important abdominal conditions, which are discussed in Chapter 9 . Fortunately, CT without oral contrast provides substantial information about many other abdominal conditions.
Abdominal Aortic Aneurysm
AAA is defined as dilatation of the aorta to a size greater than 3 cm in the abdomen. Risk factors for AAA include advancing age, male gender, cigarette smoking, atherosclerotic disease, and family history of AAA. Diabetes, female gender, and black race are negatively associated with AAA. However, advancing age is the greatest risk factor, and no patient can be considered at no risk unless aortic imaging has been performed. Risk of acute aneurysm rupture increases with increasing aneurysm size, which correlates with increasing wall tension. Above a diameter of 5 cm in women and 6 cm in men, the risk of rupture dramatically increases. Lederle et al. reported the 1-year incidence of rupture was 9.4% for AAAs of 5.5 to 5.9 cm, 10.2% for AAAs of 6.0 to 6.9 cm, and 32.5% for AAAs 7 cm or greater. Brown et al. reported the rupture rate for aneurysms of 5.0 to 5.9 cm to be 1% per year in men and 3.9% per year in women. The rupture rate increased dramatically for both men and women with aneurysms of 6 cm or greater, reaching 14.1% and 22.3%, respectively. A rapid increase in the rate of aneurysm expansion also may predict rupture, although the mean difference in expansion rates between AAAs destined to rupture and those that do not rupture is small—an increase of less than 0.5 cm per year.
The presentation of AAA rupture can be extremely variable. The classic presentation includes sudden abdominal and back pain, associated with hemodynamic collapse. This may be the most common presentation of AAA rupture, but the majority of patients who have AAA ruptures with immediate hemodynamic instability never reach an emergency physician’s care. Of those with AAA ruptures, 50% die before reaching a hospital, and the overall mortality is greater than 50%. In those patients surviving until hospital arrival, the clinical presentation is often more subtle. Fewer than half of those patients reaching the emergency department have the classic features of abdominal and back pain with hypotension. In one study of patients dying from AAA rupture, only 20% of patients presented with abdominal and back pain with hypotension. Hypotension with back or abdominal pain occurred in 60%.
Although a pulsatile abdominal mass is classically present, in several studies, the presence of a palpable pulsatile mass is unreliable. The sensitivity of abdominal palpation has been estimated from 15 studies of asymptomatic patients screened with ultrasound. For smaller AAAs, between 4.0 and 4.9 cm, the sensitivity of palpation is only around 50%, although these aneurysms have a low rate of rupture. The sensitivity of physical exam reaches 76% with an aortic diameter of 5 cm and greater, meaning that as many as one in four large AAAs in a size range at risk of acute rupture might be missed by palpation. The negative likelihood ratio for palpation in AAAs greater than 4.0 cm is only 0.51 with a 95% confidence interval (CI) of 0.38 to 0.67, making the absence of AAA by examination too unreliable for clinical use. The sensitivity of palpation is also compromised by increasing abdominal girth. The sensitivity of physical examination in the setting of acute rupture is not well studied. Marston et al. reported a pulsatile mass to be present in only about 60% of 152 patients with a ruptured AAA. In those patients who were initially misdiagnosed, only 26% had a pulsatile mass. It is possible that examination is even less sensitive once the AAA has ruptured. As a consequence, physical examination must never be used to rule out AAA rupture.
Historically, AAA rupture has been misdiagnosed initially in 30% to 60% of cases or diagnosed after substantial delay. Many patients present with surprising apparent hemodynamic stability and lack of anemia. Gaughan et al. found that initial hemodynamic stability was associated with delay in diagnosis, perhaps reflecting the supposition among physicians that AAA rupture would ubiquitously result in shock. Common alternative diagnoses in cases of actual ruptured AAAs include renal colic, urinary tract infection, spinal disease, diverticulitis, and gastrointestinal hemorrhage. Although these misdiagnoses may be less common today in an age of ubiquitous CT, the frequency of misdiagnosis in the past highlights the insensitivity of history and physical examination for the diagnosis. In one study of patients dying from AAA rupture within 2 hours of presentation, the median presenting systolic blood pressure was 110 mm Hg, the median presenting heart rate was 71 bpm, and the median presenting hemoglobin was 9.0 g/dL—all values that might be mistakenly interpreted as relatively reassuring. Remember that the median value is the 50th percentile, so half of patients had laboratory or vital sign values less ominous than those listed above. That these values occurred in patients who ultimately died within 2 hours of emergency department arrival emphasizes that seemingly reassuring vital signs and laboratory values offer little prognostic reassurance. This also stresses the urgency of rapid imaging, as diagnosis using CT with oral contrast can result in delays as great as 140 minutes. Although the time before diagnosis or death in some cases of AAA rupture can be greater than 24 hours, 12.5% of patients die within 2 hours of emergency department presentation without intervention. In any individual patient, the moment of hemodynamic collapse cannot be predicted, and all urgency should be taken in making the diagnosis. Patients who are hemodynamically unstable at the time of surgery have a higher 30-day mortality rate than those who are not, emphasizing the need to identify patients with AAA rupture before physiologic collapse.
Imaging for Suspected Ruptured Abdominal Aortic Aneurysm
Because of the potential for rapid and sudden hemodynamic collapse, imaging for suspected AAA must be performed as rapidly as possible. Simultaneous with all diagnostic imaging should be patient resuscitation and surgical consultation. As we discuss in detail later, ultrasound is a sensitive modality for the presence of AAA, although it is insensitive for detecting aneurysm rupture. CT without any contrast, or with IV contrast if time allows, is usually definitive. X-rays play little role except in evaluating for other immediate surgical causes of abdominal pain such as free peritoneal air and should be avoided when possible to prevent dangerous delay.
Ultrasound for Abdominal Aortic Aneurysm
Ultrasound is considered highly sensitive for the detection of AAA. Multiple studies have investigated ultrasound in the hands of emergency physicians. In the context of acute abdominal pain, the presence of AAA on emergency physician–performed ultrasound has a very high positive predictive value, allowing rapid triage to operative care without further imaging. Ultrasound measurements of aortic diameter performed by emergency physicians correlate closely with measurements made using CT or MRI, differing by only around 4 mm. Knaut et al. reported that aortic measurements by emergency physicians closely estimated those from CT, differing by less than 1.5 cm at the superior mesenteric artery (SMA) and 1 cm at the aortic bifurcation in 95% of cases. Emergency physicians can perform the examination rapidly, in fewer than 5 minutes in most cases. The examination is simple to learn as well, with physicians becoming proficient after brief training. In one study of an abbreviated ultrasound examination performed in patients with suspected ruptured AAA, the sensitivity of ultrasound for detection of AAA is reported as 97%, with few indeterminate results in the hands of radiologists. Tayal et al. found that ultrasound performed by emergency physicians was 100% sensitive (95% CI = 89.5%-100%) with 98% specificity (95% CI = 92.8%-99.8%). In their study, 10 of 27 patients were taken to the operating room without further imaging following emergency physician ultrasound diagnosis of AAA. Costantino et al. similarly found that emergency medicine resident–performed ultrasound was 94% sensitive (95% CI = 86%-100%) and 100% specific (95% CI = 98%-100%).
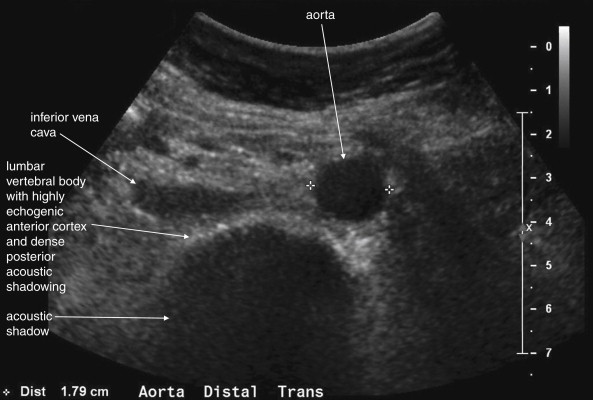
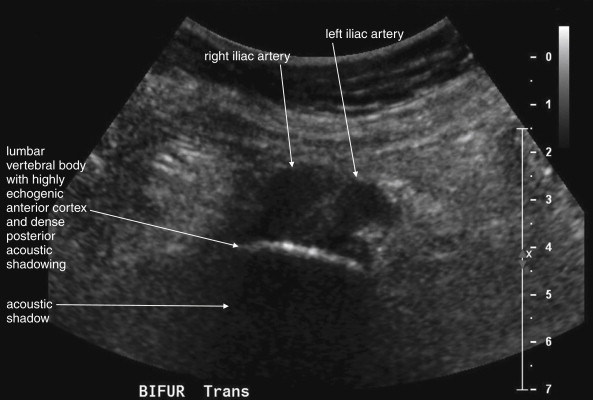
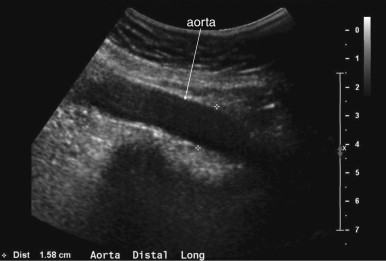
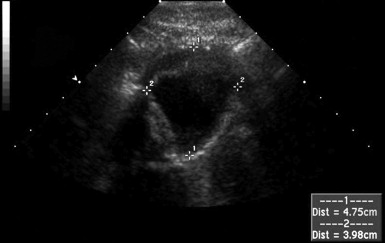
The normal appearance of the aorta is simple to recognize on ultrasound (see Figures 11-4 to 11-6 ). The aorta occupies a uniform location anterior to the vertebral column, which serves as a reliable landmark. On transverse imaging, the aorta usually lies slightly left of the midline of the vertebral column. The inferior vena cava usually lies just to the patient’s right of the aorta. The anterior bony cortex of the vertebral body appears as a bright or highly echogenic curve, deep to the aorta. Deep to this curve, dense black acoustic shadowing occurs, making the remainder of the vertebral body invisible. Anticipation of this appearance of the vertebral body prevents mistaking the vertebral body for a large aortic aneurysm. The abdominal aorta should not exceed 2-3 cm. Aortic aneurysm is defined by a diameter exceeding 3 cm, although rupture is unlikely below 5 cm in women and 6 cm in men.
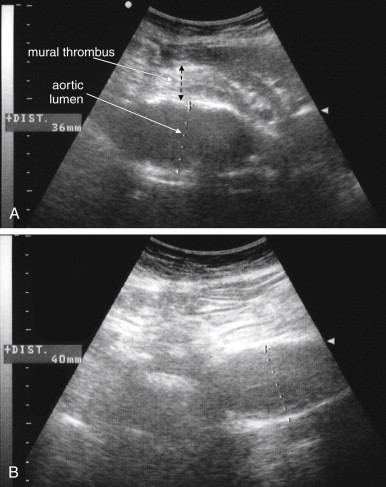
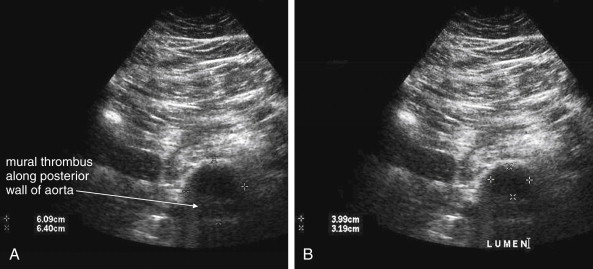
Several pitfalls in the ultrasound evaluation of AAA should be considered. First, the reported sensitivity for AAA rupture is a stunning 4%, according to a single study published in Radiology in 1988. In this study, an abbreviated ultrasound examination was performed during the ongoing resuscitation of the patient, and only the periaortic region was examined for blood. Blood collecting in other abdominal areas such as Morison’s pouch might have been missed by the abbreviated exam. The retroperitoneum, where aortic hematoma might collect following a contained aortic rupture, is generally not well-evaluated with ultrasound. The sensitivity for AAA rupture today with modern ultrasound machines is unknown but must be assumed to be poor until proven otherwise. The second pitfall is undermeasurement of the aortic diameter because of the presence of circumferential mural thrombus (see Figures 11-7 to 11-10 ). Thrombus is common within aortic aneurysms and reduces the fluid-filled lumen visible on ultrasound. Although thrombus is usually visible on ultrasound, if it is not recognized, the fluid-filled channel may be measured without inclusion of the thrombus, underestimating the aneurysm size. A third pitfall is failure to image the abdominal aorta along its entire length from the xiphoid process to the umbilicus. Aortic aneurysms may occur anywhere along this length, although infrarenal aneurysms are most common. An additional pitfall is measuring the aorta in a longitudinal direction but failing to sample along the maximal diameter (see Figure 11-11 ). If a section of the aorta parallel to the long axis but not along the midpoint is measured instead, undermeasurement of the diameter may occur. Despite these concerns, in one study, longitudinal measurements correlated better with CT than did transverse measurements. Finally, failure to recognize a nondiagnostic ultrasound resulting from overlying bowel gas can result in a missed aneurysm.
Contrast-Enhanced Ultrasound
Several new studies suggest a possible solution to the poor sensitivity of ultrasound for the diagnosis of ruptured AAA. Contrast agents originally developed for echocardiography provide a means of visualizing both the aortic lumen and extravasation of blood. A variety of agents approved by the U.S. Food and Drug Administration are available. They shared a common feature of containing highly reflective microscopic bubbles that are extremely echogenic on ultrasound. Studies in the context of acute abdominal trauma and acute AAA rupture suggest improved sensitivity for extravasation of blood. Contrast agents can be injected at the bedside with a single hand bolus, followed by immediate performance of ultrasound. Catalano et al. reported the technique in the assessment of eight patients with ruptured AAA and visualized retroperitoneal hematoma and extravascular contrast leak in seven of the eight. The sensitivity has not been confirmed in larger series. Contrast-enhanced ultrasound has also been used to monitor aortic aneurysms for endoleak (discussed later in this chapter) following endovascular repair. In this setting, it has excellent sensitivity (100%) and specificity (93%) compared with multislice CT angiography. It outperforms color duplex ultrasound, which has a sensitivity of only 33.3% and specificity of 92.8%. Ultrasound contrast agents can be used in patients with renal insufficiency, severe iodinated contrast allergy, or hemodynamic instability preventing use of CT scan.
Computed Tomography for Ruptured Abdominal Aortic Aneurysm
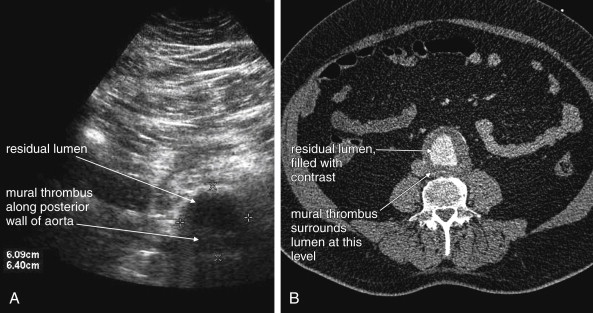
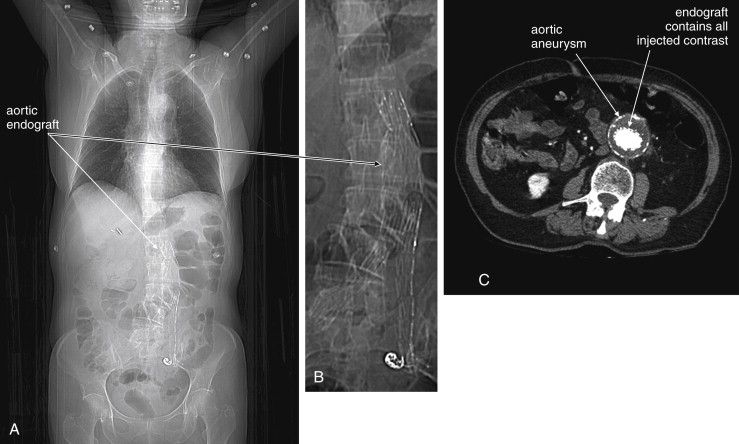
CT scan without IV contrast can diagnose the presence of AAA and AAA rupture with high sensitivity and specificity ( Figures 11-12 to 11-16 ). An AAA is defined by an aortic diameter 3 cm or greater, but ruptures are rare in aneurysms of this size. The aorta is readily identified as a circular structure in cross section, anterior to the spine. On unenhanced CT, high-density retroperitoneal hematoma can be seen in the perinephric retroperitoneal space, sometimes displacing the kidney anteriorly. Without IV contrast, blood shares the same density as the kidney and psoas muscle and can obscure the lower density fat plane that normally separates these two structures from one another and from the aorta. In addition, a high-attenuation crescent within the aortic wall or within mural thrombus predicts rupture. Without IV contrast, the crescent is considered to be “high attenuation” if its Hounsfield density exceeds that of unenhanced blood in the aortic lumen. If time allows, CT scan with IV contrast provides more anatomic definition to allow open surgical or endovascular intervention ( Figures 11-17 and 11-18 ; see also Figures 11-13 and 11-14 ). IV contrast also identifies ongoing hemorrhage at the time of CT. Oral contrast should never be used when AAA rupture is suspected, because it introduces an unacceptably long delay before imaging.







Alternative Imaging Modalities for Abdominal Aortic Aneurysm Rupture
Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) can be used to diagnose AAA with excellent sensitivity and specificity, but the delay before imaging is unacceptably long in most institutions using this modality. Noncontrast CT should always be preferred over MRI, because it is highly sensitive and specific for AAA and AAA rupture. Catheter-based formal angiography can be used to diagnose AAA with or without rupture, although in most institutions this has been replaced by ultrasound or CT imaging for diagnostic purposes, as angiography is both time-consuming and invasive. Plain radiographs play virtually no role in the diagnosis of AAA and should be avoided whenever possible, because they may introduce unacceptable diagnostic delay. Rarely, the calcified outline of an aortic aneurysm may be seen on x-ray, but the sensitivity is unknown and plain x-ray should not be used for this diagnosis ( Figure 11-19 ). An exception to this rule is in the unstable patient in whom a competing diagnosis such as perforation of a hollow viscus is being considered. In this case, an upright portable chest x-ray to assess for pneumoperitoneum can be rapidly performed (see Figure 11-1 ).

Angiography
Angiography is the historical gold standard for the diagnosis of AAA, aortic dissection, and mesenteric ischemia. Today, the wide availability of CT angiography has relegated catheter-based angiography to a purely therapeutic role following a diagnostic CT in most centers (discussed in more detail in Chapter 16 ). Angiography can be used for the treatment of even ruptured AAAs using endovascular stents. Because the stent must be sized carefully to prevent occlusion of branch vessels and to ensure that a leak does not occur at the margins of the stent, CT with IV contrast and multiplanar reconstructions is usually required (see Figures 11-17 and 11-18 ). Aortic dissection can also be diagnosed with angiography. Under angiographic guidance, stenting of dissections or fenestration of the intimal flap to restore blood flow to an occluded true lumen is sometimes used as a therapy. Angiography can be used to diagnose and treat mesenteric ischemia (see Figure 11-35 later in the chapter). Tissue plasminogen activator or similar thrombolytic agents can be infused through a catheter. Vascular stents can also be placed to restore blood flow. The urgency to restore bowel perfusion cannot be overemphasized. Surgical consultation should be performed even if interventional radiology is the intended initial therapeutic approach. Death from delay in reperfusion can occur; increased time to surgery is strongly associated with poor outcome.
Imaging of Patients With Prior Endovascular Abdominal Aortic Aneurysm Repairs: Endoleaks
Following endovascular repair of AAAs, an endoleak may occur ( Figures 11-20 and 11-21 ). An endoleak is defined as the continued flow of blood into the aneurysm sac. Endoleaks are graded as one of four types. Type 1 endoleaks occur immediately, at the time of placement of an endovascular graft, usually because of mis-sizing of the graft, such that the proximal or distal ends of the graft are not sealed against the aorta. This requires placement of a larger graft, because type 1 endoleak leaves the aneurysm sac subject to systemic vascular pressures with continued risk of acute rupture. Type 2 endoleaks result from retrograde flow of blood through collateral vessels into the aneurysm sac (see Figure 11-21 ). Common examples include filling of the aneurysm sac from lumbar perforating arteries. Type 2 endoleaks are often observed without treatment, because they do not subject the aneurysm sac to full systemic vascular pressures and are at relatively low risk of rupture. Type 2 endoleaks that do not resolve spontaneously can be treated with angiographic embolization. Type 3 endoleaks occur when a tear in the fabric of the graft occurs or if separation occurs between grafts consisting of multiple modules. This type of leak is relatively rare but leaves the aneurysm sac subject to systemic vascular pressures, requiring replacement of the graft because of continued risk of aneurysm rupture. Type 4 endoleaks occur from leaks through pores of the graft material and usually do not require repair.
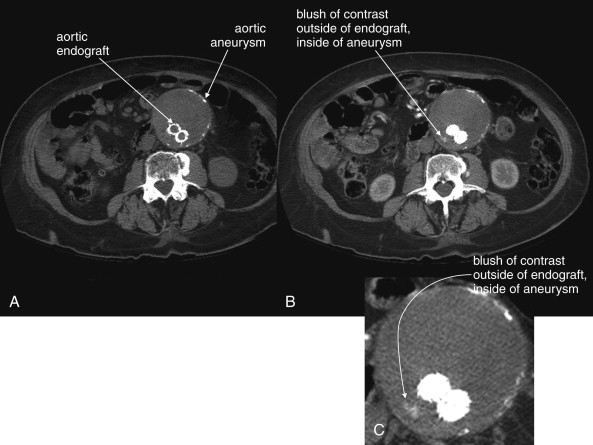
Definitive diagnosis of an endoleak can be made with IV contrast–enhanced CT (see Figures 11-20 and 11-21 ). Without IV contrast, the presence of an endoleak will go unrecognized on CT, because leaking blood will have a similar Hounsfield density to thrombus outside of the stent but contained within the aneurysm sac. With the addition of IV contrast, blood flow within the aneurysm sac outside of the stent is evident. Because retrograde filling of the aneurysm sac is often slow (e.g., with type 2 endoleaks described earlier), some endoleaks can be difficult to detect on arterial-phase CT with IV contrast. Additional delayed images (acquired approximately 5 minutes after initial contrast injection) may be required. Some authors have argued against the need for delayed phase images, because most endoleaks detected with this technique resolve spontaneously without intervention. Contrast-enhanced ultrasound can be used to assess for endoleak in patients with contraindications to IV contrast for CT. Contrast-enhanced ultrasound performs favorably compared with CT. MRI can also be used to assess for endoleaks, because graft materials are MRI compatible. Contraindications to gadolinium contrast are discussed elsewhere in this chapter.
Aortic Dissection
Dissection of the thoracic aorta presenting primarily with abdominal pain is rare, occurring in only 4.6% of cases in a large international registry. However, mortality is increased in these patients, resulting from a delay in diagnosis because of atypical presentation. Although type B aortic dissections (those affecting only the descending aorta) account for 37.5% of aortic dissections, only 1.3% of all aortic dissections affect solely the abdominal aorta. Risk factors for aortic dissection include collagen vascular diseases such as Marfan’s and Ehlers-Danlos syndrome, but these account for a minority of cases (2.9% of type B dissections) in the International Registry of Acute Aortic Dissection (IRAD), and a history hypertension is likely the greatest risk factor, occurring in 80%. Pregnancy and the postpartum state are also risk factors, likely as a consequence of increased collagenase production during this time. Use of stimulant drugs such as cocaine can also result in aortic dissection. Although the classic description of aortic dissection includes sudden tearing pain radiating to the back, the clinical presentation can be quite variable. Sudden pain is common (80% to 90%), as is migratory pain (25%). In the International Registry of Acute Aortic Dissection, among type B dissections presenting with primarily abdominal pain, tearing pain was described in 25%, sharp pain in 44%, pressure-like pain in 33%, and burning pain in 6%. The latter descriptors might mislead emergency physicians who are commonly taught to seek “tearing” pain as a clue to aortic dissection. The age distribution for aortic dissection is skewed toward older patients, with a mean age of 65 years and with 42% of type B dissections occurring in patients 70 years or older. Patients with a personal or family history of connective tissue diseases must be suspected of dissection at a younger age.
Unlike aortic aneurysm rupture, which frequently leads to hemodynamic instability, aortic dissection can present without any substantial derangement of hemodynamics. Although hypertension is a risk factor for the development of aortic dissection, patients may present with normotension (28%), hypertension (69%), or hypotension (3%). Initially, aortic dissection is a disease process involving only the intimal layer of the aorta. A tear in the intima develops, and blood typically undermines this layer, creating a flap that extends distally in the direction of blood flow. Proximal extension may also occur. Extension into branch vessels is the greatest threat, because it can disrupt perfusion to vital organs including the kidneys, mesentery, and bowel (21% in cases of abdominal aortic dissection); spinal perforating arteries (2.7%); and extremities (25% to 30%). Proximal dissection can result in disruption of the aortic valve with acute valvular insufficiency. Dissection into the pericardium can occur, resulting in pericardial tamponade.
In none of these former cases does blood loss external to the aorta occur, accounting for the frequent relative hemodynamic stability of patients with aortic dissection. This also accounts for the low sensitivity of chest x-ray for the diagnosis of thoracic aortic dissection and naturally for abdominal aortic dissection. In traumatic thoracic aortic injury, violation of all layers of the aorta may occur, leading to extravasation of blood and formation of a mediastinal hematoma, which can in turn lead to widening of the mediastinal silhouette on chest x-ray. In contrast, nontraumatic dissection may occur in an aorta of normal caliber and may be restricted to an intimal injury without formation of a mediastinal hematoma. This can result in a normal mediastinal silhouette on x-ray, occurring in as many as 20%, according to a large international registry. Luker et al. reported that only 25% of chest x-rays were prospectively interpreted as suggesting aortic pathology in cases of acute thoracic dissection.
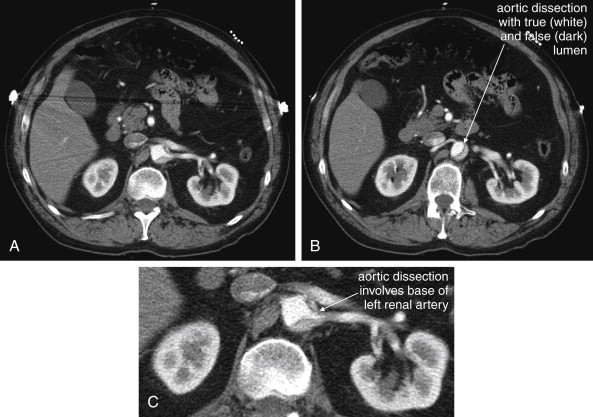
Classic examination features such as pulse deficits are relatively rare in type B dissections, occurring in only about 20% to 30% of patients with type B thoracic or isolated abdominal aortic dissections. The classic finding of a differential blood pressure between upper extremities is of unknown sensitivity and specificity. In a study of asymptomatic emergency department patients with a prior history of hypertension, 18% of patients had differences in the blood pressures obtained in the upper extremities exceeding 10 mm Hg. This suggests that the finding is nonspecific, so its use as a screening tool would result in unnecessary diagnostic imaging in many patients. The sensitivity is also likely to be very low. If the intimal flap does not involve an extremity branch of the aorta, neither pulse deficits nor differential blood pressure might result. Reliance on this physical examination finding to prompt definitive imaging would result in failure to diagnose aortic dissection in many patients. Common features that should prompt evaluation for aortic dissection include sudden onset and migratory pain, which has been found to be a frequent, though not ubiquitous, feature. Abdominal pain in concert with back or chest pain is worrisome. The presence of unilateral neurologic features also can suggest stroke from dissection involving the carotid arteries. Vertebral artery dissection can result in posterior circulation symptoms. Ischemic peripheral neuropathy occurs in a small minority of patients (2.2%). Occlusion of spinal perforating arteries can result in neurologic deficits, including bilateral limb paralysis or sensory loss. Sometimes these symptoms can be transient, and they may be mistaken for patient malingering, sciatica, or spinal cord compression. Dissection of the mesenteric arteries can result in simultaneous mesenteric ischemia in as many as 5% of cases, and renal failure can result from dissection of the renal arteries in up to 13% (see Figures 11-25 and 11-26 ). Limb ischemia (7%) may also result from abdominal aortic dissection involving the iliac arteries.
Screening for aortic dissection using D-dimer measurement has been advocated by some authors, who have reported sensitivity of 93% to 100%. A meta-analysis suggested a sensitivity of 94% (95% CI = 91%-98%). However, other authors have reported sensitivities of 88% to 92%, with false-negative results reported in patients with thrombosed false aortic lumens. Moreover, the small size of most published studies results in wide 95% confidence limits. The lower confidence limits of sensitivity reported are as low as 67% to 85% in some studies; for this reason, some authors continue to raise concerns that D-dimer testing is too unreliable for clinical use, given the high mortality of aortic dissection.
Imaging for Aortic Dissection
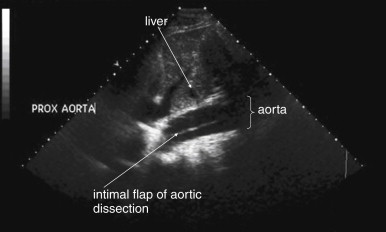
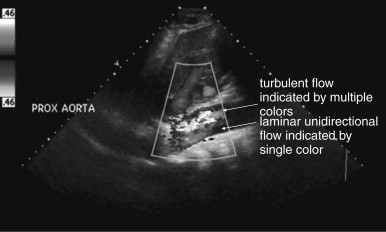
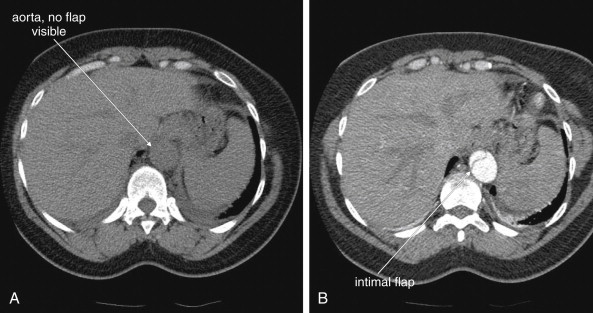
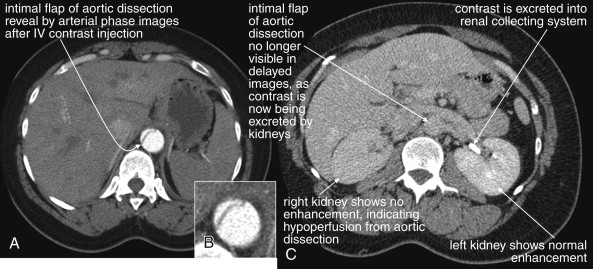
Because the clinical presentation of AAA rupture and abdominal aortic dissection can overlap substantially, diagnostic imaging for aortic dissection should begin with bedside ultrasound to assess for AAA. Ultrasound can sometimes visualize the intimal flap of dissection ( Figures 11-22 and 11-23 ). However, the sensitivity is relatively low, around 70% with use of B scan and color Doppler techniques. Contrast-enhanced ultrasound has been reported to be 97% sensitive compared with CT, although the technique is not widely used. CT without IV contrast cannot rule out aortic dissection, because the intimal flap typically shares the same density with blood in the true and false lumens ( Figure 11-24 ). Rare case reports document aortic dissection on noncontrast CT, based on displacement of calcified atheromatous plaques or high attenuation clot within the false lumen, but the sensitivity of these findings is unknown and should not be relied upon. Administration of a rapid bolus of IV contrast should be considered critical to the diagnosis. Thus if a noncontrast CT is first performed to evaluate for AAA and is negative, CT with IV contrast must be performed if possible. With IV contrast, the intimal flap is highlighted as an unenhanced line separating the true and false lumens of the aorta (see Figure 11-24 ). A normal aorta is circular in cross section, filling uniformly with bright contrast. When an intimal flap is present, the circular cross section appears divided into two lumens. These may be unequal in size. Both lumens may enhance with contrast, or one or both lumens may be thrombosed and fail to enhance ( Figure 11-25 ). In addition to initial arterial phase images, delayed images are usually obtained to assess the perfusion of organs such as the kidneys. Failure of the kidneys to enhance on delayed images indicates hypoperfusion related to the dissection and may require emergency surgery or intravascular techniques to fenestrate the dissection flap and restore blood flow ( Figure 11-26 ).
MRI or MRA is also highly sensitive and specific for abdominal aortic disease, including aortic dissection. In patients with contraindications to iodinated contrast for CT, such as renal insufficiency or iodinated contrast allergy, MRI or MRA is a reasonable alternative if its performance can be timely. Typical MRI or MRA for aortic dissection requires approximately 25 minutes to complete, compared with fewer than 6 minutes for CT. This does not take into account the limited availability of MRI outside of normal business hours in many centers, with additional time often required for technicians to arrive. Three-dimensional MRA with injected gadolinium contrast is usually used to demonstrate the intimal flap. In patients with renal insufficiency at risk for gadolinium-associated complications (discussed at the end of this chapter), MRI without IV gadolinium can be performed to assess for aortic dissection. New sequences that require no contrast material perform favorably compared with gadolinium-enhanced MRA. These protocols include steady-state, two-dimensional, gradient–recall echo ; two-dimensional turbo spin echo; and two-dimensional, balanced, steady-state free precession.
Mesenteric Ischemia
Mesenteric ischemia is not a single disease but a family of related disorders. These include acute and chronic occlusions of mesenteric arteries and acute, subacute, or even chronic occlusions of portal and mesenteric veins. Bowel ischemia can also be a consequence of small-bowel obstruction and intestinal volvulus. Vascular thrombosis most often afflicts patients at advanced age; however, rarely, conditions such as vasculitis, hypercoagulable states, or atrial fibrillation may underlie acute thrombosis in young patients. We review here the mechanism and the presentation for each of these disease states, recognizing that these diseases are rare enough that large studies with good methodology are generally lacking.
Acute Mesenteric Artery Occlusion
Acute mesenteric artery occlusion and ischemia can occur from in situ thrombosis or embolic events. Atrial fibrillation is likely the most common cause, accounting for 95% of cases in one prospective study. Dissection of mesenteric vessels can complicate aortic dissection or rarely may occur in isolation. The superior mesenteric artery (SMA) is the most commonly affected vessel in the setting of atrial fibrillation (see Figures 11-31 to 11-35 ). Occlusion of the SMA may be more common than previously believed. In a population-based study using autopsy results, the incidence was 8.6 per 100,000 person years, with a cause-specific mortality of 6 in 1000 deaths. Suspicion of intestinal ischemia before death was noted in only 33%, possibly reflecting a variable presentation not fitting the classically described signs and symptoms.
Classically, acute mesenteric ischemia from arterial occlusion is characterized by sudden and severe abdominal pain without significant abdominal tenderness. This is often described as “pain out of proportion to exam.” This may be a misleading myth that could result in misdiagnosis or delayed diagnosis in many patients. In a retrospective review from Wake Forest University, 63% of cases had a delay of greater than 24 hours from symptom onset until treatment, reflecting the difficulty of the diagnosis. Of 77 cases presenting with acute mesenteric ischemia, 64% were described in their initial emergency department record as having peritonitis–suggesting that exam findings were severe, not mild. In a second study of 43 patients with mesenteric ischemia, 48% had diffuse tenderness, and 52% had peritonitis by exam. One explanation is that these are late findings and patients often present after a delay of many hours from onset of ischemia. In any case, mesenteric ischemia should never be ruled out because the physical examination demonstrates too much tenderness.
Lab abnormalities associated with mesenteric ischemia include substantial leukocytosis, elevated anion gap, elevated lactate level, and hyperphosphatemia, but small studies on the topic limit the value of these findings in ruling out a potentially lethal process. Ritz et al. found that marked leukocytosis and elevated serum lactate occurred in 93% of patients with acute mesenteric ischemia and were prognostic of mortality. Lange and Jackel found serum lactate to be elevated in 100% of 20 patients with mesenteric ischemia, although the specificity was only 42%. Merle et al. found a serum lactate level of greater than 5 mmol/L to be associated with death within 72 hours. High inorganic phosphate levels have long been reported to be associated with mesenteric ischemia, based primarily on animal studies. Phosphate is believed to originate from sloughing intestinal mucosa. The sensitivity has not been studied in well-designed human trials but is inadequate for screening in case series. May and Berenson reported elevations in only 25% of cases of intestinal ischemia. Gorey and O’Sullivan reported hyperphosphatemia in only 15% of 65 patients with extensive intestinal ischemia, leukocytosis in 65%, and metabolic acidosis in 67%.
Pitfalls in laboratory screening can occur. Although marked leukocytosis can suggest acute mesenteric ischemia, this is likely a late finding that may occur after frank bowel necrosis has occurred, in which case patient prognosis is poor. Thus patients with normal white blood cell counts should not be dismissed as not having mesenteric ischemia. Patients with significant leukocytosis may be mistakenly suspected of infectious or inflammatory conditions of the abdomen, such as diverticulitis, abdominal abscess, or appendicitis. Although these patients undoubtedly would undergo additional observation or diagnostic imaging, the absolute time criticality of the diagnosis of ischemia might be missed. For example, observation of several hours in the case of SMA thrombosis can result in complete loss of the small bowel, with high morbidity and mortality.
When considering the screening sensitivity of laboratory tests, it is extremely important to remember the methodologic limitations of most studies on the subject, which are usually small, retrospective reviews. In addition, mesenteric ischemia is a spectrum of disease including partial and incomplete vascular occlusions. The elapsed time from the moment of vascular occlusion until emergency department presentation, as well as the degree of bowel hypoperfusion, may affect the sensitivity of laboratory testing and diagnostic imaging.
Imaging for Mesenteric Ischemia
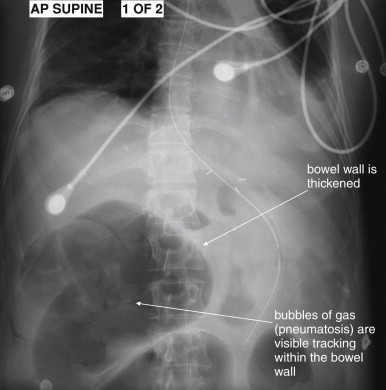
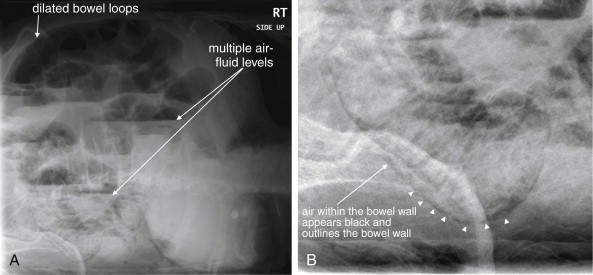
In any patient with suspected acute mesenteric ischemia, immediate surgical consultation and definitive imaging must be performed. Plain radiographs play a limited role in the diagnosis. In retrospective reviews of patients ultimately diagnosed with mesenteric ischemia, plain films are both insensitive and nonspecific. In one study of 187 patients, 35% had normal plain films and 42% showed signs of small-bowel obstruction. Common plain film abnormalities in acute mesenteric ischemia include an appearance of intestinal ileus, which should come as little surprise given that intestinal motility would be expected to cease in the absence of intestinal blood flow ( Figures 11-27 and 11-28 ; see Figure 11-3 ). Findings in one small study included air–fluid levels (84%), dilated bowel loops (48%), thickened and unchanging loops (20%), gastric distension and gasless abdomen (12%), and small-bowel pseudo-obstruction (8%). Findings of ileus may give false reassurance that no critical abnormality is present in the abdomen. The finding of intestinal pneumatosis is likely a relatively rare and late finding, occurring after the onset of intestinal necrosis (see Figures 11-3, 11-27, and 11-28 ). In one study, only 4% of patients with mesenteric ischemia had pneumatosis on x-ray. Although pneumatosis should prompt immediate surgical consultation, reliance on this finding likely results in diagnosis at such a late time that exceedingly high morbidity and mortality result. Ironically, in addition to being insensitive for mesenteric ischemia, pneumatosis is not specific for the diagnosis, occurring sometimes from a variety of less emergent causes (see Figures 11-43 and 11-46 ). As with other syndromes of acute abdominal pain, an upright portable chest x-ray is reasonable to detect pneumoperitoneum resulting from intestinal perforation (see Figure 11-1 ). A negative chest x-ray must be followed by immediate definitive imaging. Abdominal plain films should not be routinely obtained but should be considered in patients too unstable to undergo CT.