Fig. 1
Representative images of a dynamic whole body acquisition of a control rat after i.v injection of 15 MBq 18F-fluoride (mid-rat coronal slice 6–8 seconds after injection, maximum-a-posterior projections 120–180 seconds after injection, and 3000–3600 seconds after injection
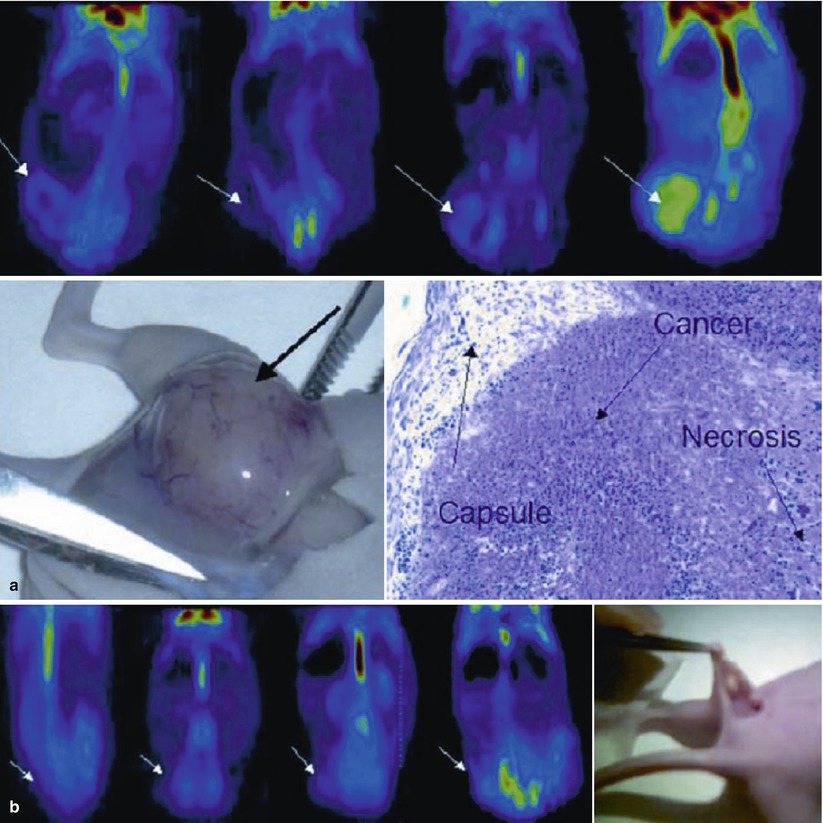
Fig. 2
(a) Coronal slice of a positive PET scan 2, 5, 14, and 20 days after inoculation; necropsy and histology (haematoxylin-eosin) showed a cancer mass. (b) Coronal slice of a negative PET scan 2, 5, 14, and 20 days after inoculation. Macroscopically, a subcutaneous mass was detectable. Histology showed a granuloma at the site of injection
On this base, Schnöckel et al. validated an easy method with 18F-SA-PET to quantify renal function in three groups of rats with normal renal function, acute renal failure and chronic kidney damage, comparing the TACs (time activity curves) obtained by a 60 min dynamic PET scan (list-mode acquisition) to the blood activity obtained by arterial blood withdrawal during the PET measurements to planar renography with 99mTc-mercaptotriglycine and to interindividual measurements as creatinine clearance and urea clearance.
The results of this experiment were satisfactory, and the accuracy of dynamic PET for the measurement of renal function turned out high. Despite that this is not closely related to a molecular imaging procedure, it can be a strong base to use it in several preclinical scenarios.
So far, this is the only published application of preclinical imaging for the evaluation of renal function.
2.2 SA-PET for the In Vivo Evaluation of Renal Cell Carcinoma in Animal Models
Despite that SA imaging is mainly applied for the evaluation of animal models of cancer, so far renal cell carcinoma has not been considered for being evaluated with preclinical imaging.
The major paper by Zisman et al. (2003) evaluated a mouse model of renal cell carcinoma expressing the carbonic anhydrase type 9 tumour antigen. In this paper the authors produced a SCID mouse model in which a clear cell carcinoma line of the chromophil type was implanted and was in vitro characterised from the histological point of view and from several molecular aspects, among which the expression of carbonic anhydrase type 9 was the most important, since it seems correlated to a poor prognosis. In fact, carbonic anhydrase type 9 has a role in the regulation of hypoxia-induced cell proliferation and may be involved in oncogenesis and tumour progression.
In the paper, no specific tracers were synthesised for the SA-PET imaging, but the animals were evaluated with a standard FDG-PET, showing an increased FDG uptake in the tumoral sites compared to normal kidneys. This in vivo demonstrates a high proliferative rate in renal tumours expressing carbonic anhydrase type 9. It is relevant to point out that, usually, renal clear cell carcinoma does not show any increased FDG uptake, while this human variant showed a significant FDG uptake, probably related to a poor prognosis.
2.3 SA-MRI for the In Vivo Tracking of Stem Cells in Animal Models of Renal Impairment
The issue of regenerating tissues that definitively lost their function due to a specific disease is serious and, in recent years, was approached by trying to inject in the impaired organ different types of stem cells. This technique is based on the principle that stem cells have a great differentiating capability and that they could differentiate into a viable specific and functional tissue once implanted in the impaired organ.
This approach gave controversial results, but it is well known that one of the major problems is the difficulty to in vivo track the stem cell and to understand their homing and their in vivo differentiation.
To do this, several imaging approaches were evaluated. One of the most effective is based on the use of SA-MR, since stem cells can be in vitro labelled with SPIO particles without compromising their function and viability. SPIO particles are retained inside the cells for a long time and are aimed to increase the MR sensitivity in detecting their localisation once injected in a living organism intravenously or within the arterial vessel afferent to the impaired organ.
Some papers were published in literature analysing the viability and homing of stem cells in animal models of renal impairment.
Hauger et al. (2006) and co-workers produced a rat model of mesangiolysis and labelled bone marrow stem cells by incubating them in vitro with SPIO. All the animals were evaluated with a SA-MR study before and after the injection of stem cells in the tail vein, and particular attention was put on the evaluation of the liver and kidneys. Furthermore, images were acquired ex vivo after the kidneys explant. The authors found a significant decrease in the signal intensity in the kidneys only in the ex vivo studies (demonstrating that stem cells have a specific homing in the damaged kidney), while the in vivo MR did not give satisfactory results, probably because the scanner sensitivity was not enough to demonstrate a small number of labelled cells.
Another interesting paper by Lange et al. (2005) is based on the same approach. The authors created a rat model of acute renal failure and analysed the homing of SPIO-labelled stem cells and the renal function trend. In this case they found that SA-MR was a feasible method to in vivo track labelled cells and that the signal loss in the damaged kidney (indicative of stem cells presence) was predictive for an increasing renal function.
Despite the potential opportunity to label iron particles to produce targeted MR contrast agents, no published applications are so far available for renal applications, and the in vivo stem cell tracking seems to be the major interest in this field.
2.4 Multimodality Imaging: Antitumour Efficacy and Feasibility of Multimodality Imaging in Renal Cancer
Recently Bult et al. (2013) proposed the intratumoral administration of holmium-166 microspheres ((166)HoAcAcMS) to locally control renal cancer growth. This new technique locally ablates renal tumours through high-energy beta particles, while the gamma rays allow for nuclear imaging and the paramagnetism of holmium allows for MRI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree