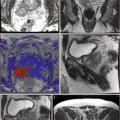
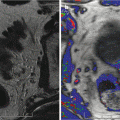
Fig. 17.1
Post HIFU necrotic calcification
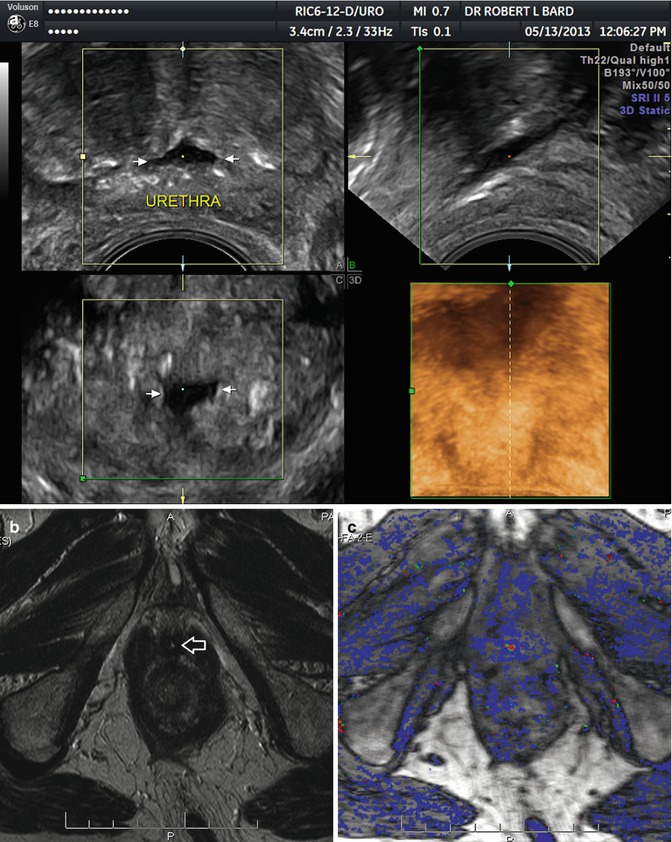
Fig. 17.2
(a) Post HIFU urethral dilation. (b) T2 MRI with thickened prostatic urethral wall (arrow). (c) DCE-MRI with periurethral hyperemia and enhancement
Tumor recurrence in the posttreatment prostate generally requires functional imaging for evaluation. 3D-PDS and DCE-MRI are usually sufficient to make a determination in the absence of a biopsy. Increased vessel density and irregular vessels in prostate cancer are present in 69 % of cases with Gleason 7 or greater tumors (Sauvain et al. 2006). External beam radical radiotherapy remains the primary treatment for patients with locally advanced or high-grade tumors in the USA as is associated with a 5-year failure rate of 35 % with Gleason scores of 6 or higher (Sartor 2002). The usual test is a PSA follow-up to determine biochemical failure (BF). This failure rate predicting tumor recurrence is less reliable than thought, and elevated PSA levels may be due to local recurrence or distant metastases. BF evaluation may be replaced by other functional parameters such as DCE-MRI and tumor vascular endothelial growth factor (VEGF) expression (Green et al. 2007). The addition of in vivo real-time volume density measurements of tumor neovascularity may prove to be of value in determining the risk of tumor recurrence noninvasively (Figs. 17.3a–d, 17.4a, b, 17.5a, b, 17.6a, b, 17.7a, b, 17.8a, b, 17.9a, b, and 17.10a, b).
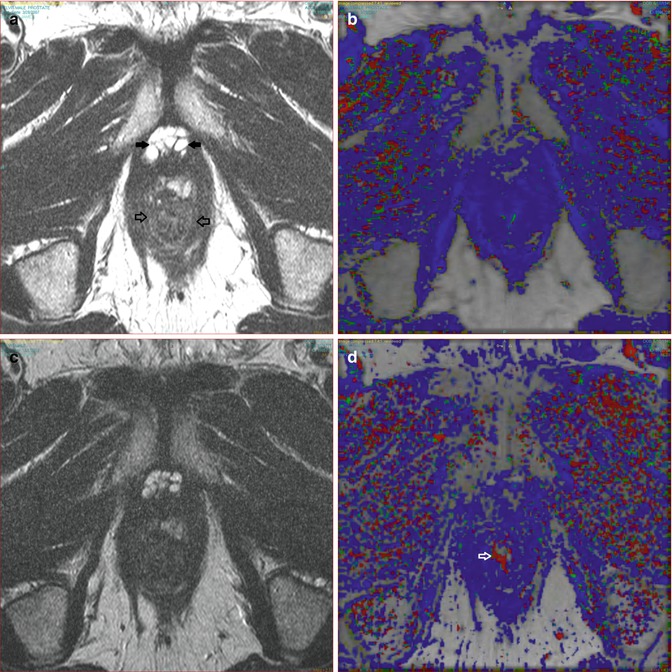





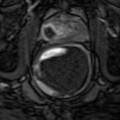
Fig. 17.3
(a) A T2 post HIFU signal loss and atrophy (open arrows). (b) DCE shows no enhancement indicating lack of recurrence. (c) T2 six months follow-up with no change. (d) DCE no interval change; note periurethral vein on right (arrow) which is not to be mistaken for recurrence and is due to vascular reorganization or recanalization following treatment changes
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree