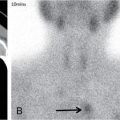
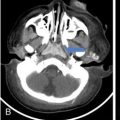
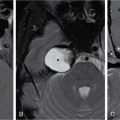
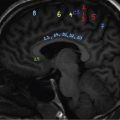
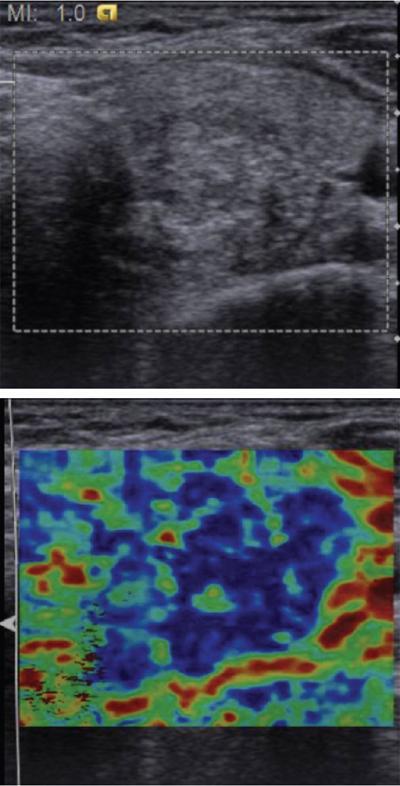
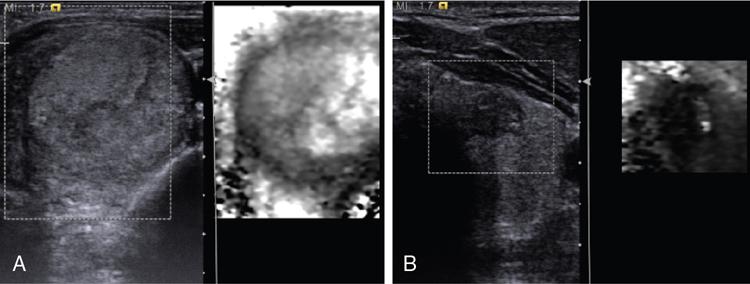
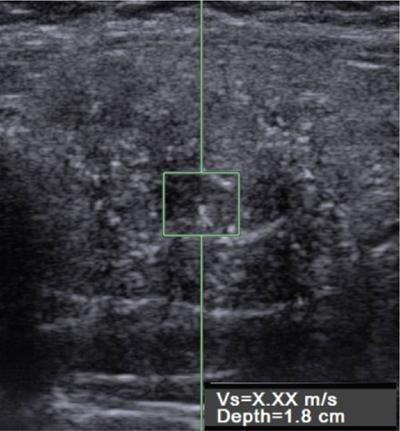
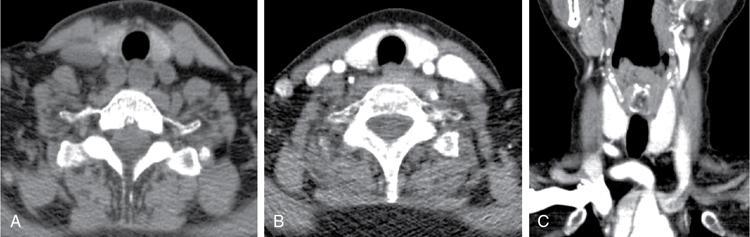
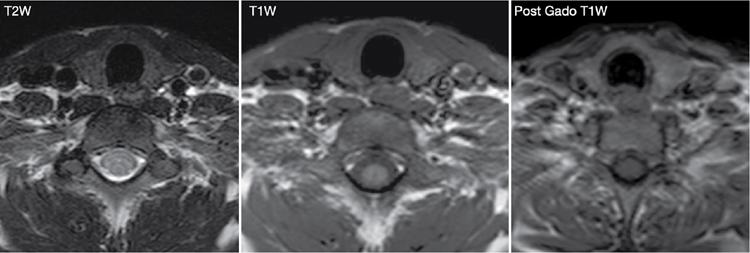
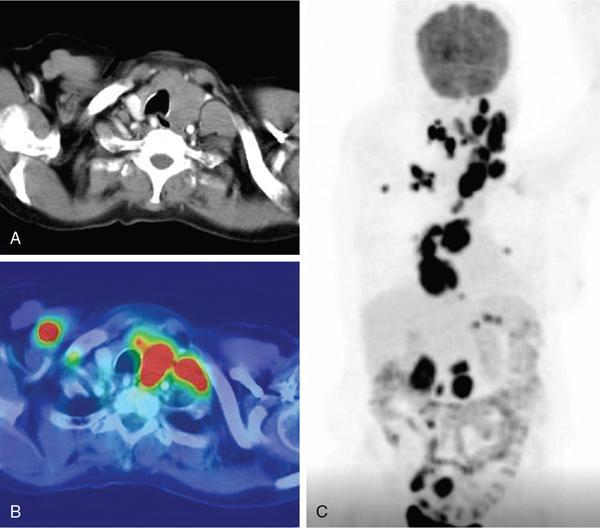
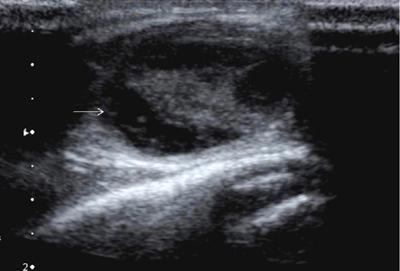
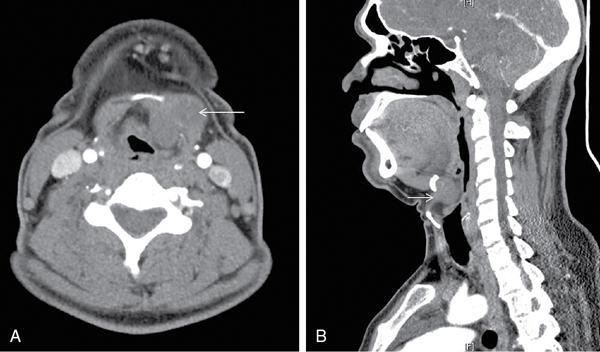
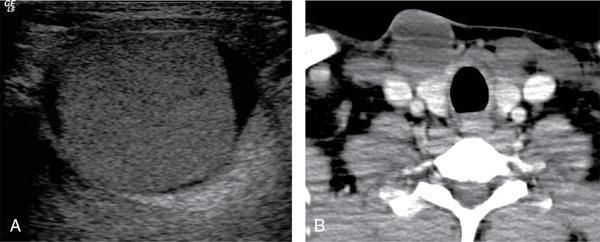
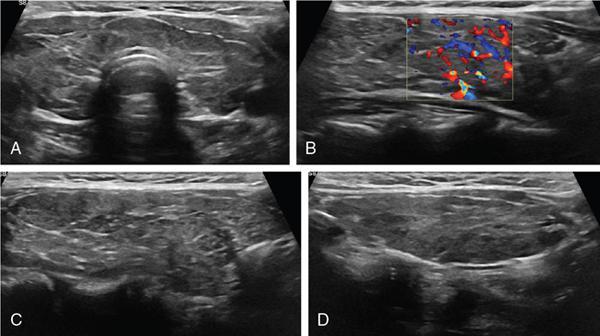
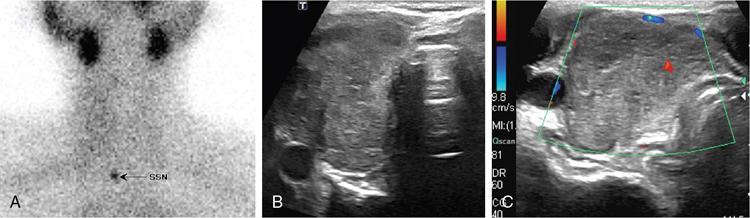
Anuradha Chandramohan, K. Madhavi, Reetu Amrita John, David Mathew Thyroid disorders are common and present with a multitude of clinical presentations to a wide range of specialities which include general physicians, endocrinologists, cardiologists, neurologists, ENT specialists, head and neck surgeons and endocrine surgeons. The clinical presentations may be due to structural or functional disorder; focal or diffuse thyroid abnormality; benign or malignant processes; symptomatic or subclinical disease. Imaging plays a central role in the evaluation and management of all these presentations. But, most importantly, the structural and neoplastic diseases of the thyroid gland. Thyroid disorders which are referred for imaging often fall into one of the following categories: congenital thyroid diseases; autoimmune or inflammatory thyroid diseases; nodular goitre; incidentally detected thyroid nodule on imaging performed for unrelated condition; staging, treatment planning and surveillance of thyroid malignancy. Ultrasonography (US) and ultrasound guided fine needle aspiration cytology (US-FNAC) are the first-line imaging investigations used for evaluation of nodular thyroid disease and clinically suspected thyroid malignancy. Other imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), Iodine-123 and Iodine-131 nuclear scintigraphy and fluorodeoxyglucose positron emission tomography with CT (FDG PET-CT) are used in specific clinical settings. In order to optimally deliver as an imaging specialist, it is vital to understand the purpose of imaging, the imaging modality of choice in a given clinical setting and how imaging findings influence the treatment decisions. In this textbook chapter on thyroid imaging, we will focus on these aspects. The thyroid is the first endocrine gland to develop in our body. The development begins at 3-4 weeks of gestational age, between the first and second pharyngeal pouches at the foramen caecum, which is located at the base of the tongue. The endodermal cells of primitive pharynx proliferate to form the thyroid diverticulum, which migrates caudally along the midline from the foramen caecum after crossing anterior to the hyoid bone and the laryngeal cartilage. Throughout its descend, the developing thyroid gland stays attached to the foramen caecum through the thyroglossal duct till it reaches its final destination in the anterior neck. The hollow thyroid diverticulum solidifies with follicular elements during its descend and divides into the right and left lobe at fifth week of gestation. Also around the same time, the ultimobranchial bodies arise from the fourth and fifth pharyngeal pouches and form the parafollicular C-cells, which secretes calcitonin, a hormone important for the calcium homeostasis. The posterio-medial portion of the thyroid where the ultimobranchial bodies fuse forms the Zuckerkandl’s tubercle, a normal variant (Fig. 3.35.1). The thyroid descent is complete by seventh week of gestation and the thyroglossal duct degenerates between the 10th and 12th week of gestation. The thyroid gland matures functionally by the 12th gestational week. The mature thyroid gland is butterfly shaped with two lateral lobes connected by isthmus. The average adult thyroid lobe measures 5 cm in length, 3 cm in width, 2 cm in thickness and the isthmus measures 1.2-1.5 cm in height. Normal thyroid isthmus is around 3 mm in thickness. Thyroid gland is a superficial gland located in anterior neck just posterior to the sternothyroid and sternohyoid muscles. Thyroid belongs to the infrahyoid visceral compartment of the neck; surrounded by the middle layer of deep cervical fascia and wraps around the cricoid and the second to fifth tracheal rings. It is separated from the oesophagus by trachea-oesophageal groove on either side, which houses the recurrent laryngeal nerve. Parathyroid glands are located posteromedial to the upper and mid portions of lateral lobes of thyroid gland. The other clinically important structures related to thyroid are laterally, the carotid sheath with its contents; anteriorly, the strap muscles and posteriorly, the prevertebral fascia and the contents of the prevertebral space. The main lymphatic drainage of the thyroid gland is to the central compartment or level VI nodes; internal jugular chain of nodes (level II-IV); retro-tracheal; parapharyngeal; superior mediastinal (level VII) and paratracheal nodes. The anatomical variants of thyroid gland include lobar hemi-agenesis; absent isthmus; pyramidal lobe which is a vestigial remnant of the distal thyroglossal duct seen as a superior sliver of thyroid issue attaches to the isthmus and Zuckerkandl’s tubercle. Thyroid gland primarily secretes thyroid hormone (T4) and small amounts of triiodothyronine (T3). These hormones play a central role in the growth and central nervous system development, regulate basal metabolic rate, body temperature, weight and affect catecholamine sensitivity of the body. Thyroid gland appears hyperdense on plain CT due to high iodine content and enhances intensely following intravenous contrast due to rich vascularity. The ultrasound is an invaluable, first choice investigation in evaluating both the focal and diffuse thyroid disorders. Ultrasound is far superior imaging modality due to its high spatial and temporal resolution. Box 3.35.1 shows the technique of ultrasound evaluation of thyroid. TECHNIQUE OF THYROID ULTRASOUND Ultrasound elastography aids in differentiating between the benign and malignant thyroid nodules by accurately estimating the differences in the tissue stiffness which is based on tissue composition. The two common techniques of ultrasound elastography are strain and shear wave elastography. Strain elastography evaluates elasticity through tissue displacement caused by compression and the tissue stiffness is displayed in colour spectrum from green (soft tissue) to blue (hard tissue) (Fig. 3.35.3). In shear wave elastography, elasticity is evaluated through the acoustic pulse of the ultrasound probe that stimulates tissues, otherwise called as Acoustic Radiation Force Impulse method (ARFI). Using ARFI technology, the qualitative assessment is done by virtual touch imaging (VTI), where the dark indicates the hard tissue and the bright indicates the soft tissue (Fig. 3.35.4). The quantitative assessment is done by virtual touch quantification (VTQ), which measures the shear wave velocity (SWV) in meters per second (m/s). Higher velocity is seen in stiff tissue and low velocities seen in soft tissues (Fig. 3.35.5). Ultrasound elastography has a role in characterization of the indeterminate thyroid nodules and has the potential for decreasing unnecessary fine needle aspirations of thyroid. However, its role is limited in nodules with calcifications, cystic nodules or nodules with significant cystic component and in differentiating follicular component of a nodule. As summarized in Box 3.35.2, ultrasound plays an important role in the evaluation of thyroid diseases. But there are few limitations to thyroid ultrasound. Firstly, ultrasound evaluation of thyroid is operator-dependent. Good training and experience are a prerequisite for optimal ultrasound evaluation of thyroid gland. While ultrasound assesses the anatomy with exquisite details, it is not possible to assess the functional aspects of thyroid gland. Also, it is difficult to accurately assess large multinodular goitres and has limitations in estimating the degree of substernal or retrosternal extensions of multinodular goitre. In malignancies of thyroid gland, ultrasound has limited value in assessing extrathyroidal disease. ROLES OF ULTRASOUND IN EVALUATION OF THYROID DISEASES There are few and specific indications for CT in thyroid diseases. These include evaluating the degree of retrosternal extension of goitre and to provide staging information in thyroid malignancy especially the extent of extrathyroidal disease, vascular invasion and prevertebral fascial infiltration in thyroid malignancy and lymphoma. CT has no role in characterizing thyroid nodules. Since iodinated intravenous contrast used for the CT impairs the thyroid uptake of radioactive iodine for 4-8 weeks, thyroid scintigraphy and therapeutic ablation with Iodine-131 must be scheduled accordingly. However, it is worth noting that contrast enhanced CT (CECT) is no longer contraindicated in these patients. In fact, when used optimally, CECT has the potential to positively impact the oncological outcome of thyroid cancer patients due to better visualization of tumour extent and the nodes prior to surgery leading to better surgical planning and surgical clearance. In the noncontrast CT, normal thyroid gland appears homogeneously hyperdense (80-100 HU) compared to adjacent neck muscles and shows intense enhancement in the postcontrast studies (Fig. 3.35.6). MRI may sometime be indicated for better evaluation of the locoregional extent of thyroid cancer. Multiplanar thin section (3-mm) images are obtained in axial, coronal and sagittal planes. The useful sequences include T1, T2, fluid sensitive sequences, postgadolinium T1-weighted images and diffusion-weighted imaging. The normal thyroid gland appears isointense to slightly hyperintense on T2-weighted images; appears slightly hyperintense on T1-weighted images compared to the adjacent neck muscles and enhances intensely following contrast (Fig. 3.35.7). Thyroid nodules incidentally detected on MRI must be evaluated with ultrasound for better characterization. Though MR spectroscopy may have a role in differentiating thyroid carcinoma from benign follicular lesion, it is not used for practical reasons. Prior studies have shown high choline peak in almost all carcinomas and the choline/creatine ratio ranged from 1.6 in well-differentiated carcinoma to 9.4 in anaplastic carcinoma. On the other hand, the normal thyroid gland and benign follicular lesions showed no choline peak. The most common reason for nuclear medicine referral for thyroid scintigraphy is for hyperthyroidism. Radioiodine thyroid scintigraphy is done to check for the percentage of iodine uptake by the thyroid gland. Following oral administration of 1 MBq of radioisotope iodine in either liquid or capsule forms, the uptake of radioisotope by thyroid gland is measured using a gamma probe after 2, 6 and 24 h. Thyroid gland can show very low uptake as in subacute thyroiditis or high uptake as in toxic nodular goitre and Graves’ disease. A focal decreased uptake or cold nodule may need further evaluation with ultrasound and ultrasound guided FNAC. Technetium 99m pertechnetate thyroid scintigraphy is used in evaluating congenital thyroid diseases in the paediatric age groups. Planar image of the neck is acquired 20 min after IV administration of Technetium 99m pertechnetate. Absence of tracer uptake can aid the diagnosis of thyroid agenesis; ectopic or incomplete thyroid descent can show as activity in abnormal location and there is intense activity in dyshormonogenesis. Whole-body radioiodine scintigraphy (WBS) using Iodine-123 or low doses of Iodine-131(I-131) is used for postoperative assessment of residual disease in patients with thyroid cancer and subsequently residual disease is ablated with high ablative doses of I-131 radio-isotope. FDG PET-CT is reserved for staging and restaging of thyroid cancer when their tumour markers are raised, but there is decreased or reduced I-131 uptake. These constitute a proportion of I-131 resistant differentiated thyroid cancers and the other aggressive thyroid cancers such as Hurthle cell carcinoma, medullary carcinoma thyroid and anaplastic thyroid carcinoma. FDG PET-CT also has a role in staging thyroid lymphoma (Fig. 3.35.8) Congenital hypothyroidism is a preventable cause of developmental delay and is usually diagnosed after routine neonatal screening. The prevalence of congenital hypothyroidism in India is one in 2640 and the overall incidence is between one in 3000 and 4000 newborns. Early diagnosis and treatment are the key to positive neurodevelopmental outcomes of this condition. Nearly 85% of congenital hypothyroidism is due to abnormal development of the thyroid gland or thyroid dysgenesis which could be from ectopic thyroid gland, athyreosis or absent thyroid gland or due to thyroid hypoplasia. These conditions are mostly sporadic in nature and two-thirds of thyroid dysgenesis is due to thyroid ectopy, which has a 2:1 female predilection. The rest 15% of congenital hypothyroidism is due to dyshormonogenesis or inborn errors in the process of thyroid hormone synthesis, and these are hereditary. The other uncommon causes of congenital hypothyroidism are central from pituitary or hypothalamic abnormalities or transient from maternal antibodies. Clinical diagnosis of congenital hypothyroidism is made using serum TSH and T4, which is widely a part of neonatal screening programs. Following the diagnosis, imaging plays an important role in determining the etiology. Ultrasound neck and thyroid scintigraphy are the two main imaging modalities that are used for this purpose. In select neonates with clinically suspected central cause MRI brain and eye examination may be resorted to. Thyroid scintigraphy is the first imaging test performed and helps in differentiating athyreosis from an ectopic thyroid gland. Thyroid scintigraphy is performed using sodium Tc99m pertechnetate or Iodine-123 (I-123). Most commonly planar images of the neck are obtained 20 min after IV administration Tc99m pertechnetate radio-isotope. The absence of tracer uptake would indicate athyreosis or absent thyroid (Fig. 3.35.9), low tracer activity indicates hypoplasia or small eutopic gland and tracer activity in an unusual location would indicate ectopic thyroid or incomplete descent of the thyroid. Other unusual causes of absent uptake could include maternal antibodies to thyrotropin receptors, iodide trapping defects and mutations of TSHb and TSH receptors. A large eutopic thyroid with increased tracer uptake suggests dyshormonogenesis beyond the stage of iodide trapping (Fig. 3.35.10). In this setting, perchlorate discharge test is performed to confirm the diagnosis. In a clinical setting of congenital hypothyroidism, thyroid ultrasound is used to compliment thyroid scintigraphy. For example, ultrasound is used in patients with absent thyroid uptake on scintigraphy in order to differentiate absent thyroid from other causes of absent thyroid uptake as described above. Enlarged thyroid gland can help in the diagnosis of dyshormonogenesis and along with decreased or absent radioisotope uptake can point to a specific type of dyshormonogenesis. Thus, ultrasound and thyroid scintigraphy must be used together as complimentary imaging modalities while evaluating congenital hypothyroidism. Ectopic thyroid is one of the top causes of thyroid dysgenesis and occurs due to abnormalities in the migration of thyroid, which can be a range from incomplete migration to over migration. Ectopic thyroid has a prevalence of 1: 100,000 to 1:300,000. The most common of ectopic locations of thyroid gland is the lingual thyroid at the base of the tongue and can present at any age from birth to sixth decade and there is 2:1 female predilection. While the earliest presentations at birth are due to congenital hypothyroidism and stridor from mass effect, the presentations at later age groups are due to palpable mass and complications such as malignancy or infection. It is important to note that 70% of lingual thyroid have absence of normal cervical thyroid. Thyroid scintigraphy is performed in all patients with suspected ectopic or lingual thyroid in order to assess for normal functioning thyroid tissue elsewhere in the neck, and this has implications in the surgical management (Fig. 3.35.11). On a noncontrast CT, lingual thyroid is characterized by a midline hyperdense mass (with a CT attenuation value of 70 ± 10 HU) at base of the tongue. Contrast-enhanced CT shows intense homogenous enhancement (Fig. 3.35.12). On MRI, both T1 and T2-weighted images show lingual thyroid as an isointense to hyperintense focus when compared to the surrounding tongue muscles and show intense enhancement following gadolinium contrast injection. Other ectopic sites in the head and neck region include trachea, submandibular region, tonsillar fossa, lateral cervical region, carotid bifurcation, pituitary gland and the eye. Apart from these sites, ectopic thyroid has been described in just about any other sites such as the axilla, heart, ascending aorta, thymus, adrenal gland, along the gastrointestinal tract and the reproductive organs. Also, ectopic thyroid can be in more than one site. Thyroglossal cyst is the most common congenital anomaly seen in the neck and present in as many as 7% of population. Thyroglossal duct cyst occurs within the embryological remnants of the thyroglossal duct which fails to obliterate. Though thyroglossal cysts can occur anywhere along the course of the thyroid descend, they are most commonly midline and closely associated with the hyoid bone. Around 20%-25% of them are suprahyoid, 15%-50% at the level of the hyoid and 25%-65% are infrahyoid in location. Around 70% of thyroglossal cysts have ectopic thyroid tissue in its wall. Clinically, thyroglossal cyst can present as a midline mobile mass that elevates with protrusion of tongue or during swallowing. Mostly, they are asymptomatic, but can present with infection or sinus formation. The goals of imaging are to diagnose thyroglossal cyst, exclude ectopic thyroid tissue within the cyst and to look for the presence of normal eutopic thyroid gland. Ultrasound is the imaging modality of choice for this purpose and thyroglossal cyst appears as a thin walled, unilocular cystic lesion in the midline of the neck in close association with the hyoid bone. Infrahyoid cyst is usually located off-midline. Proteinaceous contents from infection or bleeding within the cyst can appear as internal echoes, debris, hypoechoic or pseudo-solid echoes with no colour flow (Fig. 3.35.13). True solid component which appears as an eccentric hypoechoic focus attached to the cyst wall with internal colour flow could be due to ectopic thyroid tissue or papillary thyroid cancer. Papillary thyroid carcinoma can occur in 1% of thyroglossal cyst. Thus, ultrasound-guided FNAC is indicated to exclude malignancy in solid components of a thyroglossal cyst. The diagnosis of concurrent malignancy within a thyroglossal cyst has important management implications and can change management from simple modified Sistrunk’s procedure to include the need for total thyroidectomy, lateral neck dissection and need for subsequent radio-iodine treatment. It is important to diligently document the presence or absence of normal eutopic thyroid gland since this has implications in patient counselling for need of lifelong thyroid hormone therapy. Cross-sectional imaging such as CT or MRI is rarely indicated in the evaluation of patients with thyroglossal cyst. CT or MRI is used when there is doubt regarding the diagnosis; when underlying malignancy is suspected; or when a complex thyroglossal sinus is suspected. On CT and MRI, the thyroglossal cyst in the suprahyoid neck is usually midline and often causes remodelling of the hyoid bone. In the infrahyoid neck, the cyst is usually off midline and is in contact with the outer aspect of the thyroid cartilage and deep to the strap muscles. Infrahyoid thyroglossal cysts stretch the thyrohyoid membrane posteriorly and appear to lie in the pre-epiglottic space (Fig. 3.35.14). The cyst wall is usually thin except when there is infection or haemorrhage. The differential diagnosis for thyroglossal cyst includes other midline lesions in the neck like epidermoid or dermoid cyst; second branchial cleft cyst which are lateral along the sternocleidomastoid muscle and cystic lymph nodal metastases (Fig. 3.35.15). The key to diagnosis of thyroglossal cyst is its close relationship to hyoid bone. It is also important to note that 10% of thyroglossal cysts can recur following surgery and this is usually due to incomplete removal of involved segment of hyoid bone. A wide range of diseases affect the thyroid gland in a diffuse manner. The most common of these are the autoimmune thyroid diseases such as Hashimoto’s thyroiditis and Graves’ disease. Other disease entities that present with diffuse thyroid abnormality include subacute lymphocytitis thyroiditis, De Quervain thyroiditis or subacute granulomatous thyroiditis, acute suppurative thyroiditis, Reidel thyroiditis, amiodarone induced thyroiditis, atrophic thyroiditis and radiation induced thyroiditis. In general, thyroiditis is more common in women and is characterized by lymphocytic infiltration of the thyroid gland and subsequent destruction of thyroid parenchyma due to an autoimmune or inflammatory disease process. The clinical presentation and the need for treatment of diffuse thyroid disease is usually guided by function of thyroid gland, which varies to a wide degree during the course of the diffuse thyroid disease. Thus, patients can be hypothyroid, hyperthyroid or euthyroid. Autoantibodies to thyroglobulin and thyroid peroxidase progressively destroy the thyroid gland and cause decreased thyroid hormone production in Hashimoto’s thyroiditis. On the other hand, anti-thyrotropin receptor antibodies in Graves’ disease stimulate excessive thyroid hormone production leading to thyrotoxicosis, and this immunoreactivity underlies the mechanism for Graves’ ophthalmopathy and dermopathy. The majority of Graves’ ophthalmopathy and dermopathy are subclinical. But 3%-5% of Graves’ ophthalmopathy can be severe and sight threatening. Accurate characterization of the underlying disease entity causing diffuse thyroid disease is important because the choice of treatment, prognosis and the outcome is different for each of these entities. Other diseases that can masquerade as diffuse thyroid disease include thyroid lymphoma, metastases and multinodular goitre. Imaging does not play a primary role in the diagnosis and management of diffuse thyroid disease. But often, diffuse thyroid disease may be an incidental diagnosis during neck ultrasound performed for other indications or may be a finding in patients who undergo thyroid ultrasound as a part of goitre work up. Just like the clinical presentation and the laboratory findings, the ultrasound and Doppler imaging findings vary with the stage of the disease. Ultrasound in the initial stages of the disease shows diffuse enlargement of thyroid seen as convexity of anterior capsule of thyroid gland, generalized hypoechogenecity and increased vascularity (Fig. 3.35.16). Subsequently, as more gland destruction and fibrosis set in, margins become wavy and nodular; echotexture becomes coarse and micronodular with mixed areas of hypo and hyper echogenicity; echogenic septae of varying thickness can be seen traversing the thyroid parenchyma giving a pseudo nodular appearance (Fig. 3.35.16); regenerating nodules of residual functioning thyroid may appear as well defined, hyperechoic focus of varying size like a ‘white knight’ amidst the generalized parenchymal abnormality. In the late stage of the disease, thyroid gland can be diffusely atrophic, hypoechoic and nodular (Fig. 3.35.17). It is common to see central compartment neck nodes in patients with Hashimoto’s thyroiditis. These nodes are usually round and may lack central fatty hilum and must not be mistaken for malignancy or parathyroid lesion. Graves’ disease in particular is characterized by increased vascularity. A peak systolic velocity of 60 cm/s in the inferior thyroid artery has high specificity 90% and sensitivity of 80% in distinguishing Graves’ disease from other toxic goitres. De Quervain thyroiditis or subacute granulomatous thyroiditis can present with hypoechoic nodules within the thyroid gland. These nodules can be distinguished from thyroid malignancy by noting that the nodules from subacute thyroiditis are ‘wider than tall’ along the long axis the gland, poorly vascular and exhibit probe tenderness. Patients with pyriform sinus or fistula and those with branchial cleft fistula can present as acute suppurative thyroiditis or thyroid abscess. These patients are usually very sick and ultrasound reveals thyroid abscess with a track leading into thyroid from the piriform sinus region. Radio-isotope thyroid scintigraphy with I-123 or Tc-99m pertechnetate has a role in differentiating the causes of diffuse toxic goitres. Graves’ disease shows diffuse increase in the tracer uptake (Fig. 3.35.18) and I-131 ablation is used for the treatment of Graves’ disease. It is important to adequately treat Graves’ ophthalmopathy prior to such I-131 ablation since Graves’ ophthalmopathy is known to flare soon after I-131 ablation. Graves’ ophthalmopathy is mostly subclinical and identified on cross-sectional imaging such as CT and MRI as bilateral proptosis; symmetrical or asymmetrical bilateral medial and inferior rectus muscle enlargement in a ‘coke bottle’ fashion sparing the muscle; increased orbital fat with or without stranding (Fig. 3.35.19). The other causes of diffuse thyroid disease classically show decreased or normal tracer uptake. Except for Graves’ disease which is treated with I-131 ablation or subtotal thyroidectomy, the others are treated medically with hormone replacement or antithyroid drugs depending on the thyroid function, sometimes conservatively with just watchful waiting.
3.35: Imaging in thyroid diseases
Introduction
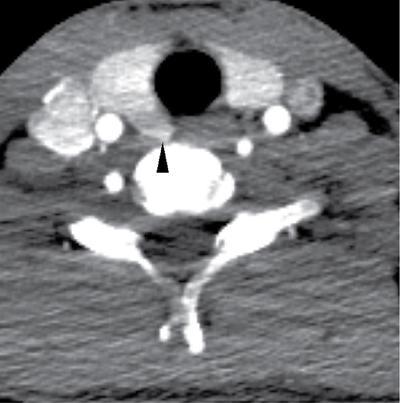
Development, anatomy and physiology of thyroid gland
Imaging modalities for evaluating thyroid diseases
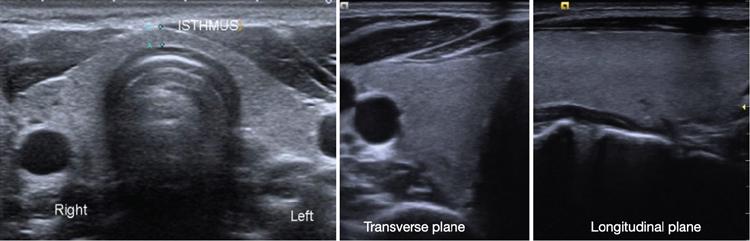
Ultrasound
Computed tomography (CT)
Magnetic resonance imaging (MRI)
Scintigraphy in thyroid diseases
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT)
Congenital thyroid diseases
Congenital hypothyroidism and role of imaging
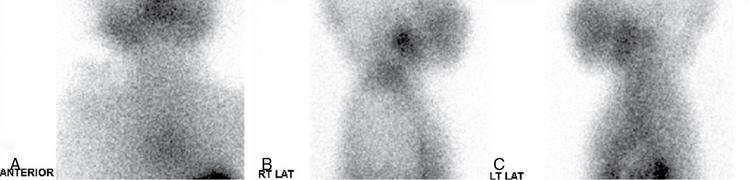
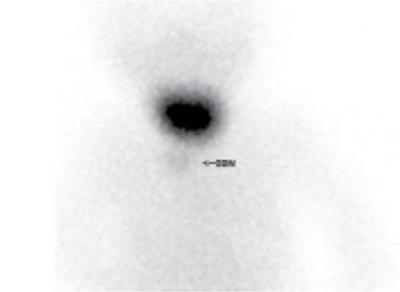
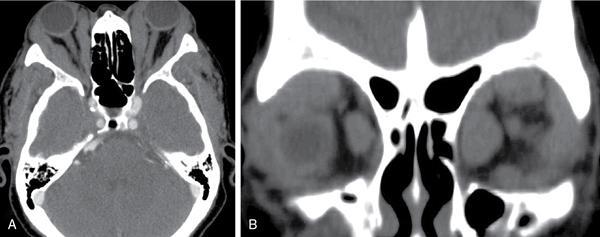
Ectopic thyroid
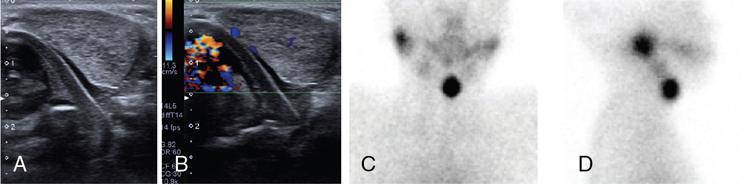
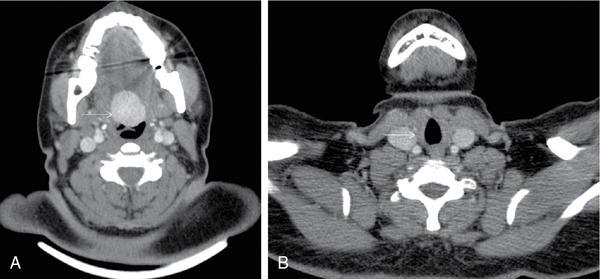
Thyroglossal duct cyst
Diffuse thyroid disease
Role of imaging in diffuse thyroid disease

Pitfalls and mimics of diffuse thyroid disease
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree