Acute kidney injury (AKI) is characterized by a decline in the glomerular filtration rate. AKI affects up to 20% of hospitalized patients, and is even more common among intensive care unit admissions. Complications of AKI are related to uremia (encephalopathy, neuropathy, pericarditis), volume overload (pulmonary edema), and electrolyte disturbances (hyperkalemia). In addition to having increased associated morbidity and mortality, patients who develop AKI may never fully recover their baseline kidney function. Imaging can play a valuable role in the work-up of AKI. This article discusses the utility of imaging in characterizing AKI in adult patients in a hospital setting.
Key points
- •
Acute kidney injury affects up to 20% of hospitalized patients, and is even more common among intensive care unit admissions.
- •
Ultrasonography serves as the first-choice imaging modality for renal assessment, and can provide valuable information, including differentiating acute from chronic kidney injury.
- •
Computed tomography is the second-choice imaging modality, because it can identify obstructive, infectious, and ischemic causes of acute kidney injury, as well as delineate disease chronicity.
- •
Magnetic resonance imaging may be useful in pregnant and young patients, because it can provide much of the same information as computed tomography without radiation exposure.
- •
Ultrasonography and nuclear medicine imaging can help evaluate acute kidney injury in transplant recipients and identify causes, including rejection, acute tubular necrosis, and drug nephrotoxicity.
Introduction
Acute kidney injury (AKI), previously known as acute renal failure, is a condition characterized by a decline in the glomerular filtration rate (GFR) over a short course (hours to days) resulting in azotemia. Although debated, multiple definitions have emerged from groups such as the Acute Dialysis Initiative, the Acute Kidney Injury Network, and the Kidney Disease: Improving Global Outcomes group. However, the clinical presentation generally consists of an increase in creatinine level and, in some cases, oliguria or anuria. AKI usually occurs in the setting of acute and chronic diseases, and is common in the hospital setting, affecting up to 20% of hospitalized patients, as well as up to 36% to 60% of admissions to the intensive care unit (ICU).
The causes of AKI are often divided into prerenal, renal, and postrenal categories. Prerenal causes of AKI are the result of hypoperfusion or hypovolemia, including decreased intake, vomiting, diarrhea, blood loss, heart failure, hepatorenal syndrome, sepsis, and less commonly renal artery stenosis and thrombosis. The most common renal or intrinsic cause of AKI is acute tubular necrosis (ATN). However, other renal causes include glomerular, interstitial, and small-vessel diseases, which may be secondary to vasculitis, infection, or drug reaction, among other causes. Postrenal causes of AKI are obstructive, such as nephrolithiasis, malignancy, and benign prostatic hyperplasia. Regardless of the cause, AKI may lead to complications caused by uremia (encephalopathy, neuropathy, pericarditis), volume overload (dyspnea, pulmonary edema), and electrolyte disturbances (hyperkalemia). These complications are associated with increased mortality in patients who develop AKI. Furthermore, some patients never fully recover their baseline renal function, and may ultimately need long-term dialysis. Of course, this common entity has a great impact on patient outcomes, length of hospital stay, and cost of care.
Although an acute increase in creatinine level is key to the diagnosis of AKI, imaging of the kidneys can provide valuable information for the work-up and management of AKI. Different imaging modalities can be used to evaluate renal anatomy, evaluate renal perfusion, rule out urinary tract obstruction, as well as assess for signs of renal inflammation and edema. This article discusses the utility of the different imaging modalities in characterizing AKI in adults, including ultrasonography (US), computed tomography (CT), magnetic resonance (MR) imaging, and nuclear medicine imaging.
Ultrasonography
The first-choice imaging modality for the kidneys in the context of AKI is US, specifically two-dimensional (2D) gray-scale US, given its ease of use, noninvasiveness, accessibility, low cost, and excellent safety profile. Advances in imaging technology have further improved visualization of the kidneys with US. Furthermore, portable US provides an advantage for imaging critically ill patients in the ICU. Although only approximately 10% of renal US examinations show abnormal findings related to AKI, these can have a significant impact on guiding patient management. A normal US also helps guide management by excluding major structural abnormalities.
Size
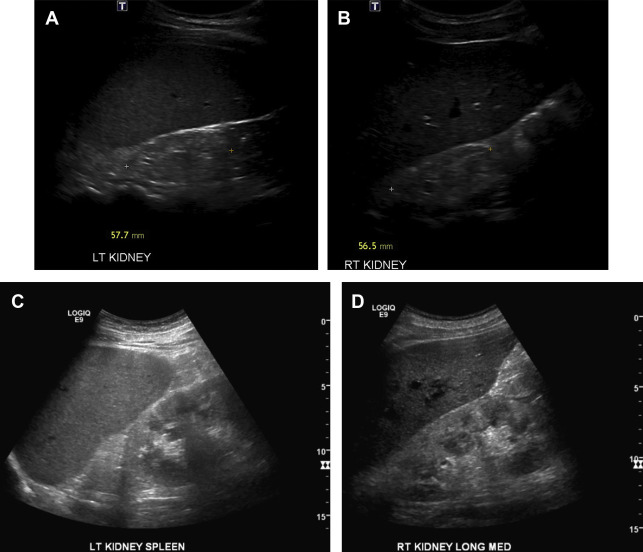
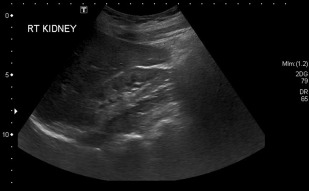
Kidney size can help differentiate acute from chronic kidney disease (CKD). Small or atrophic kidneys are often seen with CKD, in which case the patient’s renal function is unlikely to fully recover ( Fig. 1 ). Enlarged kidneys may suggest an infiltrative disease process, including lymphoma and monoclonal gammopathies. Other causes of enlarged kidneys in the setting of AKI include pyelonephritis, ATN, acute interstitial nephritis, and acute proliferative glomerulonephritis (GN). A rarer cause of an enlarged kidney is renal vein thrombosis. In renal transplant patients, an enlarged kidney may be seen in the setting of acute rejection (sensitivity and specificity >80%).
Echogenicity
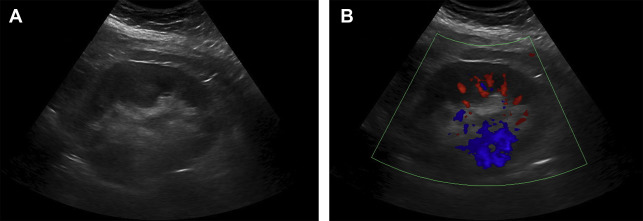
Parenchymal echogenicity of the kidneys is compared with the adjacent liver and spleen and can also help characterize AKI. Markedly increased renal cortical echogenicity is seen in CKD (see Fig. 1 ). Infiltrative diseases, such as ATN, monoclonal gammopathy, as well as proliferative and crescentic GN, show increased renal cortical echogenicity and loss of the normal corticomedullary differentiation ( Fig. 2 ). In contrast, renal parenchymal edema appears hypoechoic on US. Pyelonephritis can be seen as decreased renal cortical echogenicity with loss of the corticomedullary differentiation, but may also appear as a focus of masslike enlargement or increased echogenicity as well. More focal hypoechoic foci/fluid collections in the context of suspected pyelonephritis can be seen in the setting of abscess formation ( Fig. 3 ). The presence of a hypoechoic band surrounding the kidney may be seen in the setting of acute cortical necrosis.
Cortical Thickness
Cortical thickness can also help differentiate AKI from CKD, whereby the latter should result in decreased cortical thickness (see Fig. 1 ). Increased cortical thickness is generally seen with edema or infiltrative diseases, including ATN, acute GN, and acute interstitial nephritis. However, given that there is no established normal cutoff for cortical thickness, a baseline US scan is required to adequately detect disorder and assess for changes in patients with declining renal function.
Obstruction
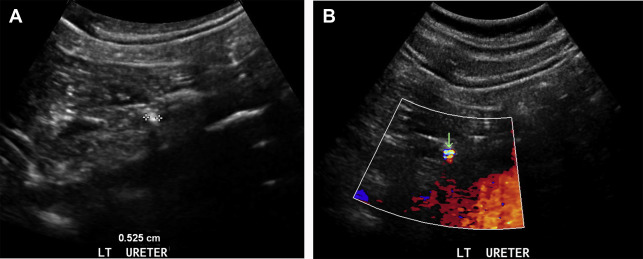
US can be used to detect signs of obstruction involving the renal collecting system, ureters, or bladder. This is one of the most important causes of AKI to diagnose by imaging because the treatment is typically centered on relieving the obstruction by catheter placement, stenting, or urinary diversion. Hydronephrosis, manifested with calyceal dilatation on US, can be seen in the context of nephrolithiasis ( Fig. 4 ), or a urinary outlet obstruction caused by an intrinsic or extrinsic cause such as a mass. Calyceal dilatation may also be secondary to urinary tract infection, physiologic dilatation in pregnancy, and in patients with diabetes insipidus. Doppler imaging may also be used to assess for obstruction, with the unilateral absence of a ureteric jet suggesting obstruction.
Renal Doppler
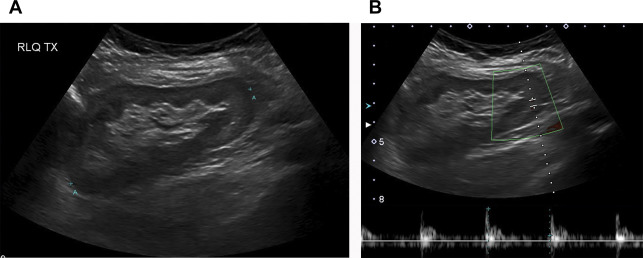
Doppler US can provide valuable information on renal blood flow. Doppler US can determine the resistive index (RI) (peak systolic velocity – peak diastolic velocity/peak systolic velocity) within the main renal arteries and smaller branches. Although some studies have found a higher RI is associated with intrarenal causes of AKI, such as ATN and vasculitis, variability caused by body habitus and an increasing RI with the patient’s age makes this measure less reliable. For example, 1 study comparing patients with ATN and prerenal AKI found that 96% of patients with ATN had an RI greater than 0.70. However, there were several false-negative examinations in patients with nephrotoxic drug-induced ATN. More recent studies have found an association between an increased RI (>0.74) in sepsis-associated AKI and post–cardiac surgery AKI, as well as persistent AKI in the ICU (RI>0.795). These findings may set the foundation for using the RI as a predictor for critical care patients at high risk for AKI. Color Doppler may also be useful in evaluating the renal parenchyma. For example, pyelonephritis typically presents with decreased color flow in the affected portions of the kidney. Urolithiasis, either in the renal parenchyma or more distally in the urinary tract, may be more conspicuous with color Doppler imaging as well because of so-called twinkling artifact (see Fig. 4 ).
Contrast
US contrast agents have been found to be safe for use even in patients with increased creatinine level and AKI/CKD. They essentially behave like red blood cells, allowing assessment of the renal vasculature. Contrast-enhanced US (CEUS) can provide an estimate of red blood cell velocity, as well as regional changes in renal blood flow to aid in the work-up of AKI. Studies in healthy patients have found CEUS to be promising in quantifying renal blood flow and assessing changes in flow, and thus may play a future role in characterizing AKI. Furthermore, a more recent study has found CEUS allows dynamic assessment of renal perfusion impairment, which may serve as a predictor of progression from AKI to CKD.
Renal Transplant
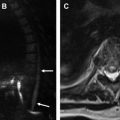
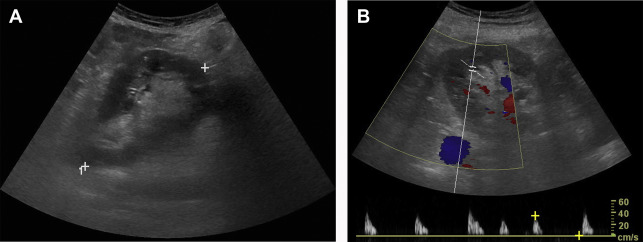
US is generally the first-line imaging test for assessment of renal transplant patients, particularly in the context of a increasing creatinine level. A diminished renal function may be the result of hyperacute, acute ( Fig. 5 ), or chronic rejection ( Fig. 6 ), among other causes that may also occur in the native kidney, such as ATN and drug nephrotoxicity. US may show a variety of different findings in the context of AKI, including increased size of the kidney, increased or decreased cortical echogenicity, loss of corticomedullary differentiation, prominent pyramids, thickening of the collecting system, and effacement of the central sinus echo complex. In the setting of chronic transplant rejection, thinning of the renal cortex and mild hydronephrosis may be seen. Furthermore, Doppler US may be used for assessment of renal transplants, with an RI greater than 0.8 being a nonspecific indicator of renal transplant dysfunction. Reversal of diastolic flow may also be seen with chronic rejection, but is much less common.
Computed tomography
Abdominal and pelvic CT can serve as a valuable second-line imaging test for assessing patients with AKI when the initial US is inconclusive. Although the topic is much debated, the use of intravenous (IV) contrast for a CT scan is avoided whenever possible in the setting of AKI. The findings of both unenhanced CT (UECT) and contrast-enhanced CT (CECT) are discussed, because contrast administration can provide relevant additional information on the renal parenchyma and corticomedullary differentiation.
Size
Similar to US, renal size can help differentiate AKI and CKD, as well as provide useful information related to infiltrative disease, pyelonephritis, ATN, renal vein thrombosis, and acute rejection in transplant patients, as described earlier.
Obstruction
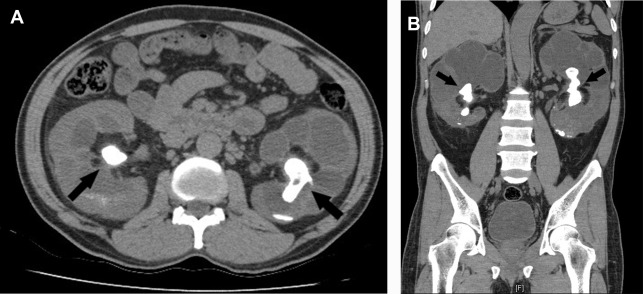
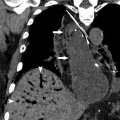
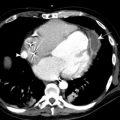
An unenhanced CT scan of the abdomen and pelvis can detect nephrolithiasis leading to obstructive AKI. This finding is especially important in the setting of a solitary kidney or bilateral obstructive nephrolithiasis ( Fig. 7 ). A CT scan can also show findings of hydronephrosis, hydroureter, as well as perinephric and periureteric stranding in the context of nephrolithiasis, even after a stone has passed. This finding may be particularly useful in the context of a negative or inconclusive/nondiagnostic initial renal US scan; for example, one that shows hydronephrosis but no definite obstructive stone.