Gastrointestinal tract perforation involving the stomach, duodenum, small intestine, or large bowel occurs as a result of full-thickness gastrointestinal wall injury with release of intraluminal contents into the peritoneal or retroperitoneal cavity. Most cases are associated with high mortality and morbidity, requiring urgent surgical evaluation. Initial patient presentations can be nonspecific with a broad differential, which can delay timely management. This article provides brief overviews of different causes of perforation. Various imaging modalities and protocols are discussed, along with direct and indirect imaging findings of perforation. Specific findings associated with different causes are also described to aid in the diagnosis.
Key points
- •
Gastrointestinal tract perforation in hospitalized patients can occur via variety of causes, including ulcers, trauma, ischemia, iatrogenic, neoplasms, infectious, and inflammatory processes.
- •
Most gastrointestinal tract perforations are surgical emergencies, but more recently initial conservative medical management has been advocated for clinically stable patients with contained perforation.
- •
Patient presentation can vary widely depending on the cause, site, and extent of injury, leading to a broad differential diagnosis. Radiologists play a critical role in narrowing down the diagnosis, which can govern medical decision making.
- •
There are several direct and indirect imaging findings of gastrointestinal perforation, which are seen to a varying degree depending on the cause of the perforation.
- •
Careful evaluation of the bowel wall enhancement pattern, length of the bowel involved, degree of the wall thickening, and the site of injury can provide important clues that can help identify the cause of the perforation.
Introduction
Gastrointestinal (GI) tract perforation involving the stomach, duodenum, small intestine, or the large bowel occurs as a result of full-thickness GI wall injury with release of intraluminal content into the peritoneal or retroperitoneal cavity. Injury can occur via variety of causes, including ulcers, trauma, ischemia, iatrogenic, neoplasms, and infectious and inflammatory processes. Clinical presentation consequently varies by the location, extent, and the cause of the injury and can range from subacute mild to acute severe abdominal pain with or without sepsis. A patient’s physiologic state can also affect the severity because immunosuppressed states, for example, can impair inflammatory response and increase the risk of free perforation. The incidences and risk factors for GI perforation from different causes are discussed later.
Given the wide range of different causes and clinical presentations, thorough history, physical examination, and laboratory findings can provide important clues that raise clinical suspicion for specific causes. Often, imaging studies, such as computed tomography (CT), are necessary for proper evaluation because many patients with GI perforation require emergent surgery. These cases are associated with high mortality (30%–50%) and require routine use of broad-spectrum antibiotics and aggressive resuscitative measures. More recently, some clinicians have advocated initial conservative management in clinically stable patients with contained perforations. Therefore, it is important for radiologists to recognize and identify the underlying cause and adequately describe the extent of the injury because these can affect management decisions. This article reviews different imaging modalities and findings that may affect management options of GI perforations from various causes.
Relevant anatomy
Although the esophagus is a part of the GI tract, this article focuses on intraperitoneal and retroperitoneal structures. Retroperitoneal structures include the second, third, and fourth duodenal segments, and the ascending and descending colon. The stomach, first segment of the duodenum, ileum, jejunum, cecum, transverse colon, and the sigmoid colon are intraperitoneal structures. The upper two-thirds of the rectum are intraperitoneal, whereas the remainder is extraperitoneal. Thus, the location of leaked intraluminal content (intraperitoneal vs retroperitoneal) can help localize the perforation site.
Some disorders preferentially affect specific parts of the GI tract. For example, the transition points between the peritoneum and the retroperitoneum along the GI tract tend to be fixed by ligaments and are prone to shear injuries in trauma. Additional disorders associated with different sites are summarized in Table 1 .
| Site | Differential Diagnosis |
|---|---|
| Stomach/duodenum | Peptic ulcer disease Necrotic/ulcerated malignancies Iatrogenic (eg, marginal ulcers, biopsy/endoscopy complication) Ingested foreign body Blunt trauma |
| Small intestine | Small bowel obstruction Ischemia Crohn disease Iatrogenic (eg, intraoperative) Penetrating trauma Malignancy Chemotherapy/radiation therapy Ingested foreign body Gallstone/enteroliths Infectious (typhoid/tuberculosis/schistosomiasis) |
| Large intestine | Appendicitis (right) Diverticulitis (left) a Ischemia Inflammatory bowel disease Malignancy Iatrogenic (eg, colonoscopy complication) Trauma |
Throughout the GI tract, the intraluminal content also varies, which can affect patient presentation. For example, perforation of the stomach, duodenum, or the large bowel may be accompanied by abundant extraluminal air and a variable amount of fluid. Small bowel perforations are generally associated with a paucity of extraluminal air with a larger amount of fluid, except in the presence of obstruction.
Regarding vascular anatomy, the stomach receives arterial supply from the branches of the celiac trunk. The duodenum receives arterial supply from both the celiac and the superior mesenteric artery (SMA) branches. The SMA also supplies the jejunum and the ileum. The ascending and transverse colon, for the most part, receive arterial supply from the SMA branches, whereas the descending and the sigmoid colon are supplied by the inferior mesenteric artery (IMA). Note that the marginal artery of Drummond provides important collateral flow between the SMA and the IMA for the colon. However, at the splenic flexure, the anastomoses between the 2 vascular territories are weak or absent and constitute a watershed area prone to ischemia or infarction.
Common imaging protocol and findings
Radiograph
Clinical presentation of GI perforation can be nonspecific. Plain radiographs are quick, inexpensive, and can help evaluate for different causes of abdominal pain, including pneumoperitoneum, which suggests GI perforation. The sensitivity of upright chest or abdomen and the lateral decubitus radiograph for large pneumoperitoneum is 50% to 90%. Supine abdominal radiographs have lower sensitivity, but some patients only tolerate this position. On upright radiographs, pneumoperitoneum is most commonly seen as a crescentic lucency under the diaphragm ( Fig. 1 A). Other radiographic signs of pneumoperitoneum are described in Table 2 . Pneumoretroperitoneum can be seen as air outlining the borders of the kidneys or the iliopsoas muscle.

| Finding/Sign | View | Description |
|---|---|---|
| Subdiaphragmatic free gas | Upright | Diaphragm outlined by the lung superiorly and by free intraperitoneal air inferiorly |
| Double bubble sign | Upright | Normal gas in the gastric fundus under the subdiaphragmatic free air |
| Decubitus abdomen sign | Lateral decubitus | Abdominal wall outlined by air |
| Rigler sign (double wall sign) ( Fig. 2 A, B) | Any | Bowel wall outlined by luminal and extraluminal air |
| Cupola sign | Supine | Lateral borders of the thoracic vertebral bodies projecting below the diaphragm outlined by the intraperitoneal air accumulating under the central tendon of the diaphragm |
| Lucent liver sign | Supine | Liver appears more radiolucent more medially because the free air collects in the anterior abdomen above the liver |
| Hepatic edge sign | Supine | Cigar-shaped pocket of air seen under the liver in the right upper quadrant |
| Doge cap sign | Supine | Triangular lucency in the right upper quadrant as the air collects in the Morison pouch |
| Football sign | Supine, pediatric | Abdominal wall outlined by air |
| Telltale triangle sign | Supine | Lucent triangle of air outlined by loops of bowel and abdominal wall |
| Falciform ligament sign (silver sign) | Supine | Falciform ligament outlined by air |
| Lateral umbilical ligament sign (inverted V sign) | Supine | Lateral umbilical ligaments outlined by air seen as a line coursing inferolaterally from the umbilicus in the pelvis |
| Urachus sign | Supine | Median umbilical ligament outlined by air seen as a midline vertical line in the pelvis |

Shortcomings of radiographs include its inability to accurately localize the injury site and low sensitivity for small amounts of air. In addition, false-positive findings can be seen with extension of air from other injury sites, such as the lungs, mediastinum, and the genitourinary system.
Computed Tomography
CT of the abdomen and pelvis is the primary imaging study for evaluating GI perforation because it has the highest diagnostic yield, with an 82% to 90% accuracy. However, because most patients present with nonspecific complaints and physical examination, the initial evaluation often involves CT of the abdomen and pelvis with intravenous (IV) contrast in the portal venous phase (PV). If there is suspicion for a more specific cause for the patient’s presentation, a more focused CT protocol can be pursued ( Table 3 ).
In general, the entire abdomen and pelvis from the dome of the diaphragm to the pelvic floor are imaged with contiguous axial sections (1.25–5 mm). Multiplanar reformats are helpful in localizing and troubleshooting equivocal findings on axial images. Although not necessary to detect pneumoperitoneum, IV contrast is almost always helpful because it increases the diagnostic accuracy for other CT findings. Patients with equivocal CT findings who are amenable to clinical monitoring may benefit from a repeat CT scan at a later time point ( Fig. 3 ). Dual energy CT may help in the detection of abnormally enhancing lesions or bowel wall by increasing the conspicuity of the iodine. Dual energy CT can also increase radiologists’ confidence in findings given the availability of iodine maps, spectral curves, iodine quantification, and virtual noncontrast images ( Fig. 4 ).

When GI perforation is suspected, water-soluble oral contrast agent is recommended because barium can cause inflammation and granulomas in the peritoneum. The use of oral or rectal contrast in the acute setting has fallen out of favor in the past decade, because several studies have shown no significant added diagnostic value and that it potentially delays diagnosis and management. The use of oral contrast also exposes patients to aspiration risk.
There are several direct and indirect signs of GI perforation on CT, with varying sensitivities and specificities depending on the cause; Table 4 summarizes CT findings of bowel and mesenteric injury for trauma as an example.
| CT Sign | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Bowel wall discontinuity | 5–34 | 100 |
| Extraluminal air | 20–30 | 95–99 |
| Abnormal bowel wall enhancement | 8–15 | 90 |
| Focal bowel wall thickening | 18–55 | 76–97 |
| Free intraperitoneal fluid | 90–100 | 15–26 |
| Mesenteric infiltration | 55–77 | 40–90 |
| Extravasation from mesenteric vessel | 17–36 | 99–100 |
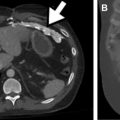
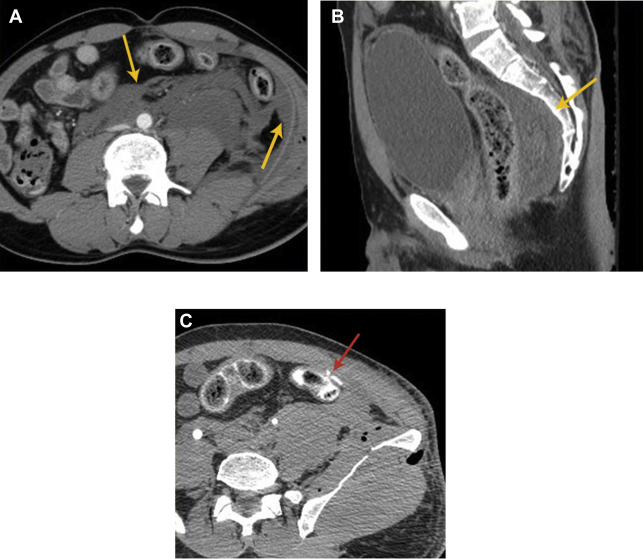
Because GI perforation requires a full-thickness wall injury, the most direct finding for perforation is bowel wall discontinuity. Wall discontinuity may be seen as a low attenuating cleft that is oriented perpendicular to the bowel wall ( Fig. 5 B). However, wall discontinuity is not always detected because the injury site may be small or seal spontaneously. Extraluminal oral contrast or air is also highly specific for perforation, with near-100% specificity ( Fig. 2 D; see Fig. 3 C). Often, the specific site of perforation can be identified by tracking the air bubbles or the extraluminal oral contrast. On supine patients, free air collects antidependently, often found in the anterior abdomen or under the diaphragm. Bone or lung windows can also help increase the conspicuity of air in the abdomen. However, these direct findings tend to have low sensitivity and are seen in less than 55% of these cases.

Therefore, the diagnosis of GI perforation often depends on recognizing indirect signs that are more sensitive but less specific. These signs include bowel wall thickening, abnormal wall enhancement patterns, mesenteric fat infiltration, and presence of free fluid. Bowel wall thickening can be caused by edema, hemorrhage, or inflammation, and it is generally defined as wall thickness greater than 3 mm or disproportionately thickened wall compared with adjacent normal bowel ( Fig. 2 E). Abnormal bowel wall enhancement includes increased and decreased/absent enhancement compared with the background bowel loops ( Figs. 6 and 7 ). Mesenteric infiltration (fat stranding) is characterized by increased attenuation and a hazy appearance of the mesentery caused by edema and hyperemia. These findings are often helpful in identifying the injured segment of the bowel. Although sensitive for bowel injury, free peritoneal fluid does not differentiate acute versus chronic or severe versus mild injuries and can also be a normal physiologic finding. However, attention to the amount of free fluid and its attenuation can be helpful in its assessment.


Mastery of the direct and indirect findings of bowel and mesenteric injury is imperative because a delay in diagnosis portends worse patient outcome. These signs of perforation in combination with the CT findings associated with specific causes, discussed individually later, are crucial in arriving at the correct diagnosis.
Sonography
Ultrasonography (US) is a fast and cost-effective imaging modality that can provide real-time dynamic information that does not use ionizing radiation. It can help provide definitive diagnosis or narrow the differential diagnosis in variety of abdominal disorders.
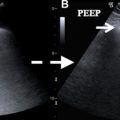
Pneumoperitoneum can be seen as echogenic reflectors with reverberation artifact that is not localized to the intraluminal space and changes with patient movement ( Fig. 1 B). Reported sensitivity and specificity of US for pneumoperitoneum range from 68% to 96% depending on the operator experience. Localization of perforation is often difficult. However, in certain cases the underlying causes, such as appendicitis, may be found. Patient body habitus, variability of bowel position, and dirty shadowing from intraluminal gas can confound the evaluation of GI perforation on US.
Acute bowel wall injury on US may be shown by bowel wall thickening, which can be seen as concentric prominence of the bowel wall layers causing targetoid appearance. Reactive inflammatory changes in the surrounding mesenteric fat can also be appreciated as increased echogenicity of the fat as well as free fluid in the peritoneum. Inflamed edematous bowel may be seen as hypoechoic muscularis propria on gray-scale images. Mesenteric or bowel wall hyperemia can be appreciated on Doppler images ( Fig. 8 ).

Ulcers
Overview
Peptic ulcer disease (PUD) is a common condition with a lifetime prevalence of 5% to 10%, accounting for nearly 150,000 hospitalization in the United States annually. It is most often caused by Helicobacter pylori infection or nonsteroidal antiinflammatory drug (NSAID) use, although other less common causes exist. H pylori causes mucosal injury by bacterial factors in combination with the host’s inflammatory response. NSAIDs cause mucosal injury by disrupting cyclooxygenase (COX)-1–derived prostaglandins, which are important in the maintenance of mucosal integrity. This mucosal injury exposes the epithelium to gastric acid, leading to ulceration and extension into the submucosal layers. However, up to70% of the patients with PUD are asymptomatic. Symptomatic patients report nonspecific complaints of mild dyspepsia that can be associated with food intake. Common complications of PUD include GI bleeding, perforation, and gastric outlet obstruction. Those patients who are smokers, consume alcohol, and are older than 60 years of age are more likely to have PUD complications.
Perforation occurs in 2% to 10% of patients with PUD, with an incidence of 4 to 14 per 100,000. It is one of the most serious complications, with a high mortality (24%–30%). Patients with perforated peptic ulcers may complain of sudden-onset severe epigastric pain that may lessen after couple of hours, but becomes generalized over time, often leading to guarding and rigid abdomen. Associated GI bleeding is common, as are shock and sepsis. Treatment of perforated peptic ulcer is most often surgical repair, which can involve peritoneal lavage, suture closure, and omental patch, along with nasogastric tube placement, IV proton pump inhibitors, and broad-spectrum antibiotics. However, in certain patients who are stable, initial conservative medical management may be pursued, especially if there is evidence of spontaneous sealing or contained perforation. Thus, it is imperative for radiologists to identify and characterize imaging findings that may lead to differential management of patients presenting with perforated ulcers.
Imaging Findings
Upper endoscopy is the study of choice for diagnosing PUD owing to its high sensitivity (>90%) and ability to biopsy suspicious lesions ( Fig. 9 ). Fluoroscopic examinations such as upper GI series have fallen out of favor because of their inferior sensitivity and the availability of endoscopy in many institutions. Ulcers on fluoroscopic studies ( Fig. 5 A) with oral contrast agent show a round or oval pocket of contrast surrounded by the smooth mound of edema and gastric folds radiating away from the ulcer. Sometimes, the Hampton line, a radiolucent line separating the oral contrast in the gastric lumen from the contrast in the ulcer crater, can be seen.

If the initial radiograph or US is unrevealing, CT is the imaging study with the highest yield when perforation is suspected. CT has low sensitivity for detecting uncomplicated mucosal ulcerations, less than 20%. CT rarely shows the ulcer as an outpouching along the gastric or duodenal wall, but more commonly shows indirect signs such as focal wall thickening and local mesenteric fat infiltration. In contrast, CT is exquisitely sensitive for evaluating for pneumoperitoneum and can effectively evaluate for complications of PUD. Note that ulcers on the posterior wall of the stomach are more likely to be associated with contained perforation because of close proximity of the adjacent soft tissues. Patients with remote abdominal surgeries are also more likely to have contained perforation because of formation of fibrous adhesions from prior surgery.
Trauma
Overview
Traumatic injuries to the bowel and mesentery are uncommon, occurring in approximately 1% to 5% and 17% of patients with blunt and penetrating trauma, respectively. Clinical evaluation of patients with trauma can be confounded by concomitant head trauma, intoxication, or distracting injuries, which render the history and physical examination unreliable. Therefore, detecting the presence of bowel and mesenteric injury on CT remains a critical task for radiologists because delays in diagnosis as short as 6 to 8 hours can cause increased morbidity and mortality because of bowel ischemia or necrosis. Most bowel injuries become clinically evident within 72 hours, manifesting with any combination of fever, tachycardia, leukocytosis, abdominal pain, or sepsis.
Three mechanisms have been proposed in the setting of blunt bowel and mesenteric injury: shear, crush, and burst. Shear injuries are caused by differential deceleration at sites of fixation, such as the ligament of Treitz or the ileocecal valve. Crush injuries result from compression of the mesentery or bowel between the abdominal wall and a fixed structure such as the spine. Burst injuries are caused by sudden increases in luminal pressures, often in the setting of abnormal underlying bowel such as ileus, obstruction, or inflammatory bowel disease. In penetrating trauma, it is imperative to follow the track of the penetrating object using multiplanar CT reformations to detect injuries and to assess whether there has been peritoneal violation. Importantly, stab wound tracks are typically narrow, whereas gunshot wounds have a broader trajectory caused by the transmission of shock waves.
Imaging Findings
Although radiographs and US are often used to quickly screen for disorders that may suggest the patient requires urgent surgical interventions, CT is the primary imaging modality for trauma evaluation, with the highest diagnostic yield.
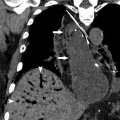
In penetrating trauma, pneumoperitoneum or pneumoretroperitoneum can be an expected finding and is not useful in the detection of GI perforation. It is also important to keep in mind that there can be pseudopneumoperitoneum from chest sources, interventions, or even bladder rupture with Foley catheter placement ( Fig. 10 ). Thus, the GI tract should be carefully evaluated for other signs of bowel injury, such as abnormal thickness, enhancement, or discontinuity of the bowel wall. The presence of free fluid in a patient with trauma without solid organ injury is concerning for bowel and mesenteric injury, with reported specificity of 15% to 26% and sensitivity of 90% to 100%. Extravasation of IV contrast material indicates active mesenteric vessel bleeding, which typically warrants urgent intervention ( Fig. 11 ). These mesenteric injuries place the associated bowel at risk for ischemia, necrosis, and perforation. Bowel wall injury from trauma is usually focal, whereas diffuse bowel wall thickening (>10 mm) can be seen in shock bowel ( Fig. 12 ). Additional injuries associated with bowel and mesenteric injury include the so-called seatbelt sign, anterior and lateral traumatic hernias, Chance fractures, multiple abdominal visceral injuries, and pancreatic injuries.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree