Fig. 22.1
(a) Virtual 3D model created using the MeVis Liver Analyzer software that enables volume measurements. (b) The software displays the hepatobiliary anatomy after virtual hepatectomy
Key Teaching Point
Pre-transplant imaging in living donors reduces operative time and the risk of interoperative injury by making the transplant team aware of anatomical variations and anomalies, allowing for volumetric measurements of the donor liver, and detecting unexpected focal or diffuse liver disease.
Conventionally, Doppler sonography is used to assess the anatomy and patency of the main vessels, such as the hepatic arterial system, portal venous system, hepatic venous system, and inferior vena cava (IVC). In many cases, multiphase CT or MRI can help delineate the vascular map and biliary anatomy prior to surgery.
Timely detection and diagnosis and prompt management of postoperative complications have resulted in improved patient outcomes. By far, the most dreaded complication is a compromised arterial vascular supply to the allograft, which may result in graft failure and occasionally, patient death. In liver transplantation, clinical findings or laboratory alterations may not be specific to any particular complication, making their interpretation a challenge for transplantation surgeons and medical services. For instance, a transient elevation in liver function test results in the early postoperative period can be seen in otherwise healthy patients; it may also be due to allograft rejection, acute diffuse liver disease, or vascular compromise. Therefore, diagnostic imaging plays a particularly crucial role during the early postoperative period [2, 14]. It is imperative that the transplantation surgeons and radiologists are familiar with the surgical procedure, including various vascular and biliary anastomoses, immunosuppressive measures, and cross-sectional imaging appearances of various reversible complications and irreversible entities that mandate re-transplantation.
Key Teaching Point
Posttransplant imaging plays a crucial role in the detection of transplant complications when the clinical picture is unclear; however, there must be familiarity with normal surgical variations versus sometimes subtle findings indicating a complication.
With the exception of postoperative fluid collections (e.g., hematoma, seroma, or abscess), post-OLT complications can be broadly classified as vascular, biliary, or parenchymal (Algorithm 22.1). Vascular complications include arterial and venous thromboses or stenoses, hepatic artery pseudoaneurysm, arteriovenous fistulas (AVFs), and hepatic infarction. Biliary complications include bile leak, biloma formation, bile duct stricture or occlusion, and cholangitis. Parenchymal complications include rejection, which has no specific imaging finding, recurrence of primary liver disease which led to transplant, posttransplant lymphoproliferative disorder, recurrent neoplasm, and metastatic disease.
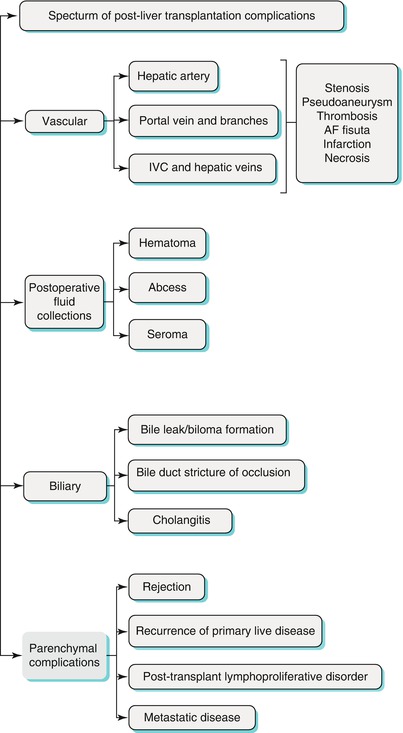

Algorithm 22.1 Specturm of post-liver transplantation complications
The primary focus of this chapter is to review the current surgical techniques used in cadaveric and living liver transplantations and illustrate the pertinent multimodality imaging findings of the common complications of OLT. Further, a diagnostic algorithm to evaluate and manage common complications in OLT patients has been provided.
Liver Transplantation Vascular and Biliary Anastomoses
OLT entails creating surgical anastomoses between the hepatic artery, portal vein, inferior vena cava (IVC), and bile duct of the donor to those of the recipient. Identifying the type and site of vascular and biliary anastomosis is essential because most postoperative complications occur at the site of anastomosis.
Hepatic Artery Anastomosis
In whole liver transplantation, the donor hepatic artery is usually an end-to-end anastomosis to the recipient hepatic artery at the bifurcation of the left and right hepatic arteries or at the takeoff of the gastroduodenal artery (Fig. 22.2a). In recipients with small or diseased hepatic arteries, arterial reconstruction can be performed using a donor conduit from the recipient aorta (aortohepatic conduits) (Fig. 22.2b, c) or viscerohepatic (e.g., spleno-hepatic conduits) [15].
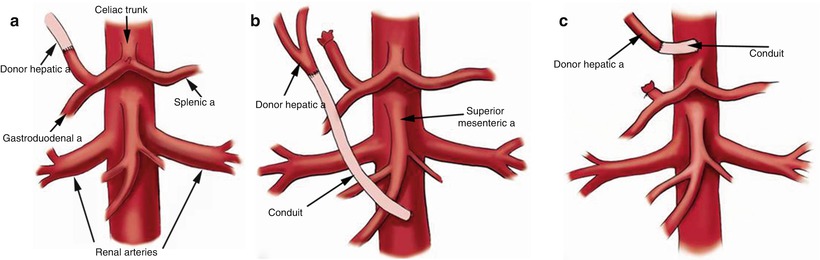

Fig. 22.2
Types of hepatic artery anastomoses: (a) End-to-end. (b, c) Aortohepatic conduits
In split grafts, including LDLT, the recipient hepatic artery is anastomosed to the donor’s right or left hepatic artery. Usually, the donor hepatic artery is too short or small and requires arterial reconstruction using microvascular surgical techniques [9, 16].
Identifying common hepatic arterial variants in potential liver donors is important because they influence donor suitability and can change the type of anastomosis. Sometimes, hepatic artery anastomoses must be performed in two parts.
Portal Vein Anastomosis
Portal venous anastomosis is also created in an end-to-end fashion between the main portal veins of the donor and the recipient (Fig. 22.3a). In cases of portal vein thrombosis or previous portal venous surgeries, graft interposition may be needed (Fig. 22.3b) [15]. In a split graft, portal anastomosis is created in an end-to-end fashion between the left or right portal vein of the donor and the recipient main portal vein [9].
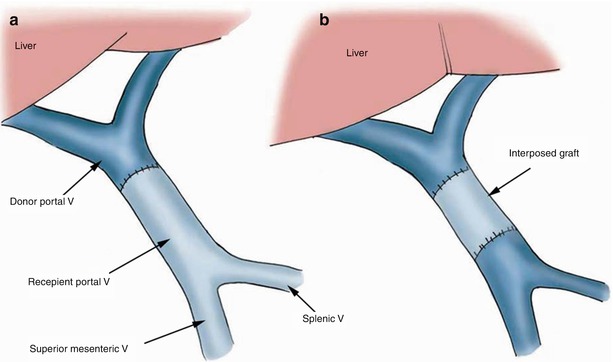

Fig. 22.3
Types of portal vein anastomoses: (a) End-to-end. (b) Graft interposition
IVC and Hepatic Venous Anastomoses
Several options exist for IVC anastomosis in OLT. The donor suprahepatic and infrahepatic IVC can be attached to the recipient suprahepatic and infrahepatic IVC, respectively, by end-to-end anastomosis, subsequent to resecting the retrohepatic portion of the recipient IVC (Fig. 22.4a). One technical variant is the piggyback technique, which involves conserving the recipient IVC by creating one anastomosis at the single graft-recipient caval junction (Fig. 22.4b) [17]. At a cavoplasty outflow connection, the graft IVC is patched directly onto an incised recipient IVC (Fig. 22.4c).
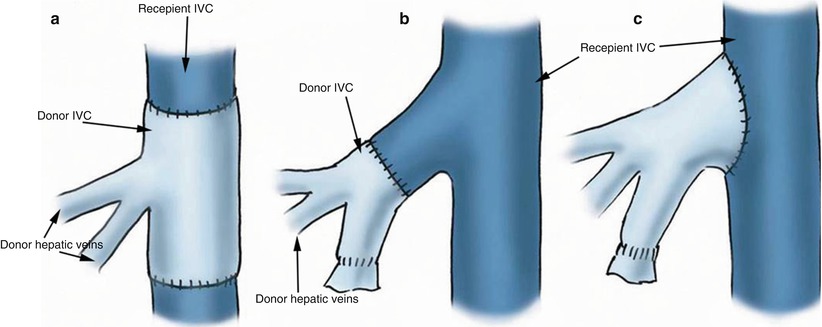

Fig. 22.4
Types of venous/caval anastomoses: (a) Intercaval connection – two anastomoses. (b) “Piggyback” connection – one anastomosis at a single graft-recipient caval junction. (c) Cavoplasty connection – patch onto recipient IVC
With LDLT, the donor hepatic vein is anastomosed to the recipient IVC. By far, the most commonly used technique is end-to-end anastomosis between the middle hepatic vein of the recipient and the donor hepatic vein [18]. However, we should be aware of the various anastomoses as many different institutions have adopted their own hepatic vein anatomosis techniques, some favoring cavoplasty and others preferring piggyback.
Key Teaching Point
There are several types of vascular anastomoses, which depend on the anatomical measurements of the donor and recipient and the type of transplant that is performed (whole liver versus split graft).
Biliary Anastomosis
Biliary anastomosis is created in an end-to-end fashion between the common bile ducts of the donor and recipient (choledochocholedochostomy), always accompanied by cholecystectomy (Fig. 22.5a). A T-tube is left in place for 3 months to facilitate cholangiography or other biliary procedures.
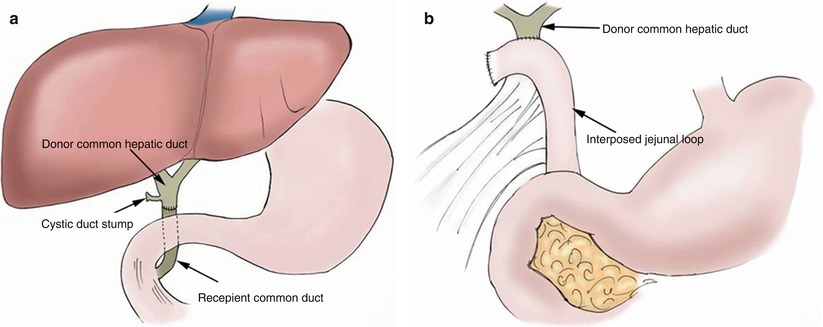

Fig. 22.5
Types of biliary anastomoses (a) Biliary-biliary: choledochocholedochostomy. (b) Biliary-bowel: choledochojejunostomy
In some situations, such as with a discrepancy in donor and recipient duct size or preexistent disease (biliary atresia, sclerosing cholangitis, or primary biliary cirrhosis), biliary reconstruction is performed using choledochojejunostomy with a Roux-en-Y limb anastomosis (Fig. 22.5b) [17].
Key Teaching Point
A cholecystectomy is always performed during liver transplantation. The presence of gas in the biliary system may be normal in OLT patients especially those with a biliary to bowel connection. In addition, sphincterotomy may be performed in the case of a biliary to biliary connection causing pneumobilia.
Immediate Postoperative Period
Changes related to surgery in the first few days after OLT can result in a range of findings, on the postoperative images, that may be perceived as abnormal. These findings do usually resolve within a few days and are due to the recent effects of surgery. These effects include the normalization of the liver from the changes of cold ischemia followed by reperfusion and manipulation of the liver from the point of donor extraction to recipient transplantation. As mentioned earlier, liver function tests (LFTs) can be abnormal in healthy patients; therefore, elevation in LFTs in the early postoperative period is not helpful to decipher abnormal imaging findings. On grayscale ultrasound evaluation, the starry-sky appearance, characterized by accentuation of portal venule walls due to decreased hepatic parenchymal echogenicity, can be seen due to the effects of stasis of fluid in the extracellular spaces and reperfusion edema [19]. Other parenchymal findings include focal increased echogenicity and heterogeneity, possibly due to contusion and/or hemorrhage [20]. Both the starry-sky appearance and parenchymal heterogeneity tend to resolve after the first few days post-OLT.
Hepatic arterial velocity can be elevated due to decreased cross-sectional vessel diameter. During the first few days after OLT, increased hepatic artery velocity can be considered a normal transient occurrence; however, these patients should undergo Doppler surveillance to ensure normalization of hepatic arterial velocity [21]. This phenomenon can occur due to a persistent high-arterial-inflow condition from portal hypertension or luminal narrowing caused by anastomotic edema. The resistive index (RI) is a measure of resistance to flow and an elevated RI may be a normal transient postoperative phenomenon and can occur in nearly 50 % of patients, resolving in a few days [22, 23]. This may be caused by vasospasm, tissue edema, increased portal flow, advanced age of donor, and prolonged cold ischemia time [22, 24–26]. A radiologist identifying a tardus parvus waveform, particularly with normal hepatic function, should request close follow-up imaging rather than diagnosing arterial stenosis in the first few days after transplant as this finding may also be caused by edema at the anastomotic site [17, 27, 28]. Similar to increase hepatic artery velocities, the portal venous velocity may be elevated due to postoperative edema and other causes, which may result in turbulent flow at Doppler US or even pulsatile flow. There is typically a return to more normal velocities and monophasic flow in the first week after transplant and eventual complete normalization of portal venous velocity after a few years [20, 29, 30]. Finally, the hepatic venous system may lose the normal triphasic waveform on Doppler US and demonstrate an abnormal monophasic pattern, which may indicate the rare occurrence of hepatic vein stenosis or more likely graft edema or compression from adjacent postoperative fluid collections [20].
In the immediate postoperative time period, a myriad of abnormal findings may be present on ultrasound and Doppler imaging as just described and can be transient, improving in the first postoperative week. If there is lack of improvement on follow-up imaging on the second or third day, further evaluation with angiography may be warranted dependent on the severity of the abnormality, the liver function status, and overall clinical state of the patient [20].
Vascular Complications
After rejection, vascular complications are the second most common cause of graft failure and mortality after OLT. They should be considered in patients with bile leak, liver cell failure, intra-abdominal bleeding, and septicemia. Early and accurate diagnosis of these complications is essential to avoid patient morbidity and mortality.
Hepatic Artery Complications
The most common vascular complications, with a reported incidence of 4–25 %, include thrombosis, stenosis, pseudoaneurysm, and AVF. Hepatic artery thrombosis and stenosis can lead to biliary ischemia because the hepatic artery is the major vascular supply to the bile ducts [8]. The portal venous blood and hepatic artery blood both contribute to the sinusoidal blood flow around the bile ducts. It is true that the hepatic artery supplies the majority of the oxygen tension, but studies have shown viability of bile ducts with oxygenation of portal venous blood with arterio-portal shunting in cases of hepatic artery thrombosis.
Biliary ischemia may, in turn, lead to a non-anastomotic biliary stricture or leak resulting in a biloma. It can also lead to hepatic failure, with subsequent necrosis and graft loss. Sepsis and gastrointestinal bleeding may also complicate hepatic artery thrombosis.
After OLT, the normal hepatic artery waveform on Doppler sonography shows a rapid systolic upstroke and a continuous diastolic blood flow. The resistive index (RI) is 0.5–0.8, calculated using the following equation: RI = (SVp−DVp)/SVp, where SVp is the peak systolic velocity and DVp is the peak diastolic velocity. The normal acceleration time (from end diastole to the first systolic peak) is less than 0.08 s [31, 32].
In the early postoperative period (<72 h after transplantation), increased hepatic artery resistance (RI > 0.8) is commonly seen, although it usually returns to normal within a few days [22]. Increased resistance is associated with older donor age and a prolonged period of ischemia [22]. It is important to remember that Doppler sonography of a tortuous hepatic artery can give falsely elevated-velocity measurements due to incorrect alignment of the sample volume angle.
Key Teaching Point
Hepatic artery complications can lead to biliary and hepatic necrosis.
Thrombosis
Hepatic artery thrombosis (HAT) is the most common and grave vascular complication of liver transplantation. It occurs in 10–40 % of patients, with most cases occurring within the first 3 months after surgery. It is most common in pediatric patients, who often require more complex anastomoses with small vessels [33–35].
The risk factors for HAT include increased cold ischemia time, ABO incompatibility, graft rejection, aorta anastomosis, multiple anastomoses, young age, technical factors, and clamp injury or kinked vessels [36].
The clinical presentation of HAT varies according to the time of presentation after surgery. Early thrombosis may cause fulminant necrosis, which requires re-transplantation; arterio-portal shunting and hyperbaric oxygen therapy are unique techniques that have been used as a bridge to re-transplantation. However, in slow developing arterial occlusion, multiple arterial collaterals may develop, leading to asymptomatic HAT [37, 38].
HAT may lead to biliary complications such as biliary ischemia or epithelial necrosis. These may be further compounded by an atypical biliary leak in non-anastomotic regions [39].
Doppler and grayscale sonography is a reliable and noninvasive imaging modality for detecting HAT. The Doppler sonography criteria include abrupt arterial signal cutoff in the extrahepatic arterial branches, with complete absence of flow in the proper hepatic artery and intrahepatic arterial system (Fig. 22.6a). However, in some cases of HAT with collateral vessel formation, intrahepatic arterial flow may be noted, with a tardus parvus waveform, which is also characteristic of hepatic artery stenosis (HAS) [28, 40].
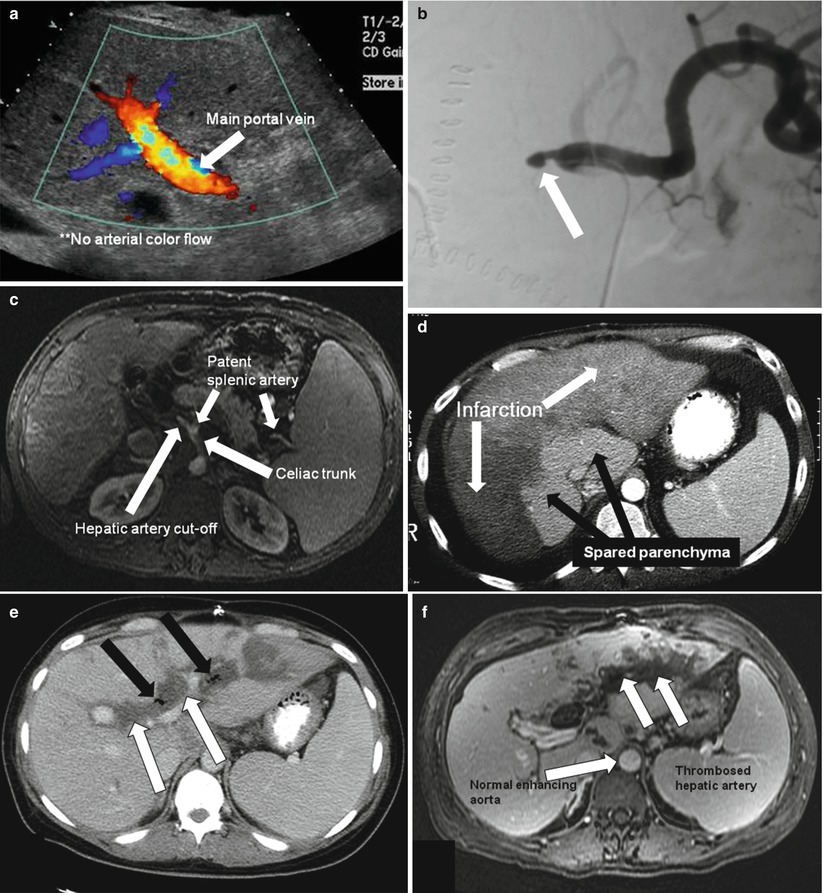
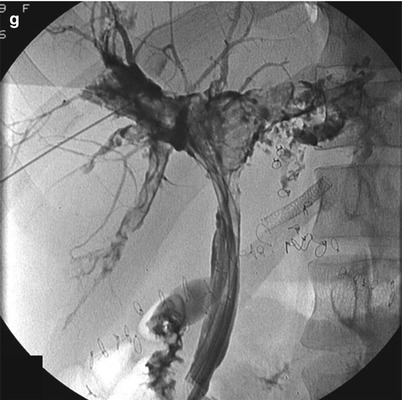


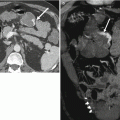
Fig. 22.6
Hepatic artery thrombosis. Color doppler ultrasonography (a) shows loss of flow in the main hepatic artery; only the portal vein. Angiography (b) shows arterial cutoff of the common hepatic artery (arrow). Contrast-enhanced MRI (c) shows hepatic artery cutoff (arrow). Contrast enhanced CT (d) shows sequela of hepatic artery thrombosis; with diffuse low-attenuation areas (white arrows) of hepatic infarction. (e-g) Biliary necrosis secondary to hepatic arterial thrombosis in a different patient; Axial contrast-enhanced CT (e), axial contrast-enhanced T1-weighted image (f), and percutaneous transhepatic cholangiography (g), demonstrate significantly dilated, thick-walled, necrotic bile ducts (white arrows) and pneumobilia (black arrow). The contrast-enhanced T1-weighted MR image also demonstrates hepatic artery thrombosis (dotted arrow in f)
The right and left hepatic artery branches should also be evaluated because a normal hepatic artery waveform obtained at the porta hepatis does not exclude a more distal hepatic artery obstruction. Tamsel et al. [41] found two cases with normal RIs and systolic acceleration times in the main hepatic artery and proximal parenchymal branches but thrombosis in the distal parenchymal branches of the hepatic artery on arteriography.
The sensitivity of conventional Doppler sonography can be further improved using microbubble contrast material. This can decrease the need for more invasive investigations such as angiography [42].
False diagnoses of HAT may result from low-flow conditions such as severe hepatic artery stenosis or low cardiac output, which yield nonvisualization of flow on Doppler sonography [43]. In addition, hepatic artery hypoperfusion can be seen in cases of so-called splenic artery steal syndrome, in which increased portal venous blood flow causes hepatic arterial vasoconstriction and preferential flow of the celiac axis to the hypertrophic splenic artery. In patients in whom no flow is identified in the hepatic artery, CT or MRA is usually required to confirm the diagnosis. The CT criterion for diagnosing HAT is the appearance of an abrupt cutoff of flow in the artery (Fig. 22.6d). CT may also demonstrate low-attenuation foci in the liver parenchyma, representing liver infarction (Fig. 22.6d). However, angiography is the definitive modality for HAT diagnosis because it shows the abrupt arterial cutoff. Severe HAS, which is treatable, may not be distinguishable from HAT on sonography or CT [44]. Angiography is indicated for a definitive diagnosis and potential transcatheter therapy. In some cases, re-transplantation is needed; however, emergent thrombectomy and arterial revascularization may be helpful [43, 45].
Key Teaching Point
If hepatic arterial thrombosis is detected on ultrasound, a CT and ultimately a conventional angiogram is almost always performed to verify the findings.
Stenosis
HAS is the second most common vascular complication. It occurs in about 5–11 % of patients at the site of anastomosis, usually within the first 3 months after surgery [46]. Early detection is important because it may develop into HAT and lead to hepatic ischemia, biliary ischemia, hepatic necrosis, and graft loss [47]. Poor surgical technique, intimal trauma, and clamp injury are risk factors for HAS. Early detection and prompt treatment are mandatory to avert graft loss [48].
HAS may be suspected when intrahepatic Doppler sonography reveals a tardus parvus waveform, which has a prolonged systolic acceleration time (≥0.08 s) and a low RI (<0.5) (Fig. 22.7a). In these instances, the main hepatic artery should be scanned to assess for focal peak velocity more than 200 cm/s, which is diagnostic for HAS [41]. An abnormal Doppler waveform can be seen in HAT, but a focal increase in peak systolic velocity is not seen. Contrast-enhanced sonography and cross-sectional imaging with angiography are helpful in these cases. The criteria for HAS diagnosis is a focal narrowing of the hepatic artery (Fig. 22.7) [43, 44]. CT angiography may be helpful in cases with a clinical suspicion of HAS even with a normal Doppler waveform due to subtle hepatic artery narrowing [2]. Treatment options include surgical reconstruction or balloon angioplasty and stent placement [44]. The appearance of focal narrowing of the hepatic artery comes with a caveat: if the donor artery is smaller than the recipient artery, an expected caliber change will be present at the arterial anastomosis. Therefore, the clinical picture of the patient and other HAS criteria should be considered.
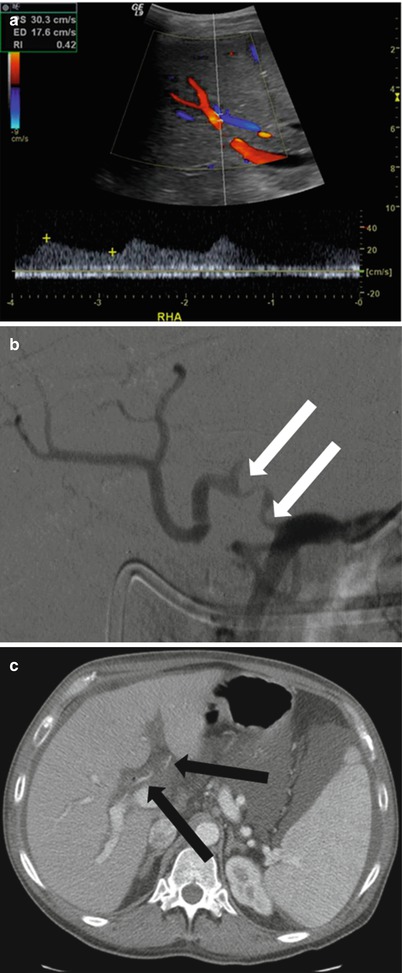

Fig. 22.7
Hepatic artery stenosis. Doppler ultrasonography (a) shows flow with a tardus parvus waveform and RI <0.5. Angiography (b) shows a segment of stenosis (arrows) at the anastomosis. Contrast enhanced CT Angiography (c) shows a markedly narrowed hepatic artery in the porta hepatis (arrows)
Key Teaching Point
The criteria for hepatic artery stenosis include RI < 0.5, tardus parvus waveform including a prolonged systolic acceleration time (≥0.08 s), or a peak systolic velocity at the area of stenosis of >200 cm/s.
Hepatic Ischemia and Infarction
Hepatic infarction is rare in otherwise healthy individuals, even with hepatic artery occlusion, because the liver blood supply is maintained by normal collateral vessels and the portal vein. However, it is a common complication of hepatic artery thrombosis in liver transplantation recipients (Fig. 22.6d) because the collateral vessels have been ligated and portal vein oxygen tension is inadequate. Hepatic infarction as a consequence of portal vein occlusion is rare [49]. Infarcted regions of the liver are prone to infection and focal abscess formation, which may result in sepsis.
Hepatic Artery Pseudoaneurysm and AVF
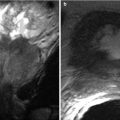
Hepatic artery pseudoaneurysms and AVFs are less common arterial complications [49, 50]. Pseudoaneurysms may occur in the extrahepatic or intrahepatic segment of the artery. Extrahepatic pseudoaneurysms occur at the anastomotic site or as complications of angioplasty. Intrahepatic pseudoaneurysms occur after liver biopsy, biliary intervention, or focal liver infection [51, 52]. They are usually asymptomatic but may present with acute shock if they rupture. Hemobilia or upper gastrointestinal bleeding may occur when a fistula forms between the aneurysm and biliary tree or the gastrointestinal tract [15, 22]. The signature pattern of a pseudoaneurysm on Doppler sonography is a turbulent multidirectional arterial flow pattern in a cystic structure at the anastomotic site or along the course of the hepatic artery. CT angiography, contrast-enhanced MRI, and conventional angiography tend to show a centrally enhancing saccular lesion arising from the hepatic artery (Fig. 22.8a–e). Extrahepatic pseudoaneurysms are treated by surgical resection, embolization, or stent placement. Intrahepatic pseudoaneurysms can be treated by endovascular coil embolization [53, 54].
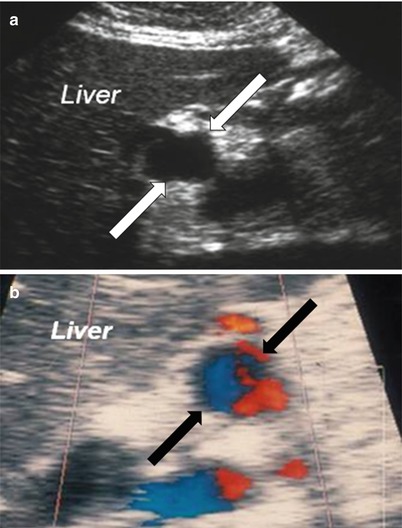
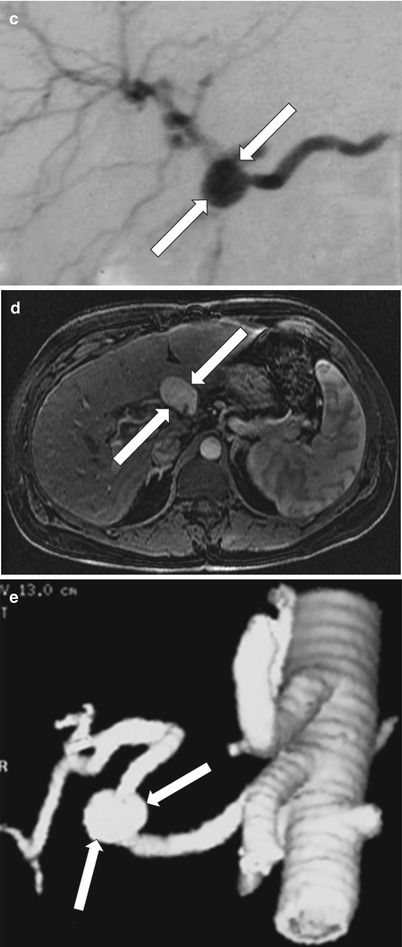


Fig. 22.8
Hepatic artery pseudoaneurysm. Grayscale ultrasound (a). Doppler sonography (b). Conventional angiography (c). Contrast-enhanced T1-weighted image (d) and volume rendering MR angiography (e) demonstrate anechoic cystic structure with a turbulent multidirectional arterial flow pattern (arrow) on color Doppler. Enhancing saccular lesion (arrow) arising from the hepatic artery is noted on conventional angiography, contrast-enhanced T1-weighted and MRA representing pseudoaneurysm at the anastomotic site of the hepatic artery
Key Teaching Point
Hepatic artery complications include stenosis, thrombosis (which could lead to ischemia/infarction), pseudoaneurysm formation, and AVF. In thrombosis, abrupt cutoff is noted. Criteria of hepatic artery stenosis include: RI < 0.5, tardus parvus waveform including a prolonged systolic acceleration time ≥0.08 s, or a peak systolic velocity at the area of stenosis >200 cm/s. Pseudoaneurysm is characterized by turbulent multidirectional arterial flow pattern in a cystic structure at the anastomotic site or along the course of the hepatic artery.
Portal Vein Complications
Portal vein complications after OLT are relatively less common and include partial or complete thrombosis, stenosis, and rarely, portal vein aneurysm [55]. Portal vein complications are usually caused by poor surgical technique, differences in the caliber of the donor and recipient portal veins, decreased portal flow or increased resistance to flow, hypercoagulable state, and history of portal vein surgery or thrombus [56]. The clinical presentation of portal vein complications varies according to the time of presentation; in the immediate postoperative period, they usually present with acute hepatic failure and edema, and in the late postoperative period, they present with signs of portal hypertension, such as esophageal variceal bleeding, splenomegaly, and ascites [56].
Stenosis
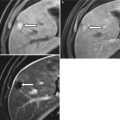
Portal vein stenosis may be seen in less than 3 % of adult recipients, whereas rates of up to 7 % have been reported in pediatric patients, with some cases progressing to portal vein thrombosis [57, 58]. In a liver graft, the normal appearance of the portal vein on color and pulsed Doppler sonography is a continuous hepatopetal monophasic flow that shows phasic variations with respiration. Turbulent flow may be a normal finding in the early postoperative period [32]. In portal vein stenosis, doppler sonography reveals focal color aliasing, with an increased peak anastomotic velocity of more than 125 cm/s or an anastomotic-to-preanastomotic velocity ratio of 4:1 (Fig. 22.9, slide b). This may be associated with poststenotic dilatation and multiple dilated collateral vessels as a result of portal hypertension [37]. CT and time-of-flight MRI are highly accurate at detecting portal vein stenosis as focal or long-segment narrowing of the portal vein (Fig. 22.9) [59]. Treatment options include percutaneous transluminal balloon angioplasty, with stent placement when needed [60].
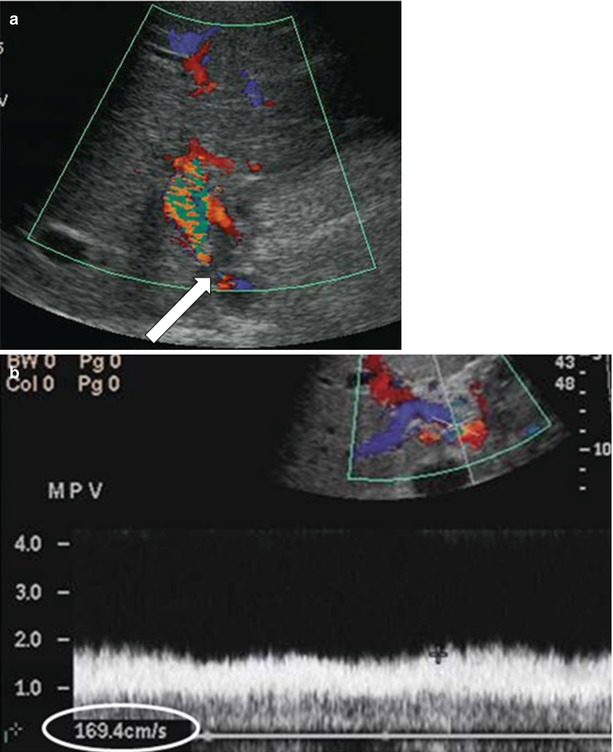
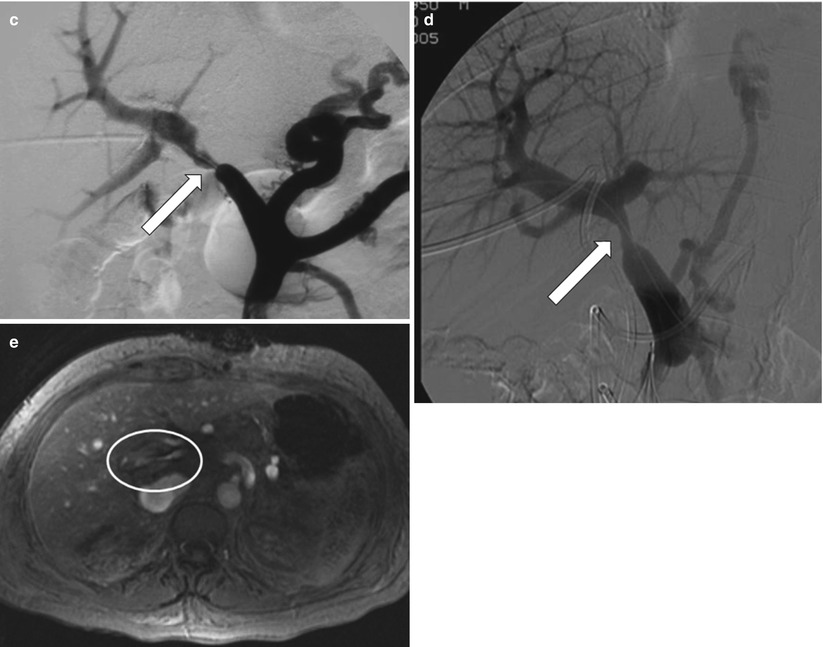


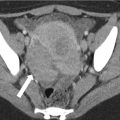
Fig. 22.9
Portal vein thrombosis. Color Doppler (a) and power Doppler (b) ultrasound examination demonstrate focal color aliasing with a velocity >125 cm/s (arrow), secondary to portal vein stenosis (arrow). Contrast portography (c, d)demonstrate marked anastomotic stenosis (arrows). 2D TOF MR Venography (e) demonstrates markedly narrowed portal vein in the porta hepatis (oval)
Thrombosis
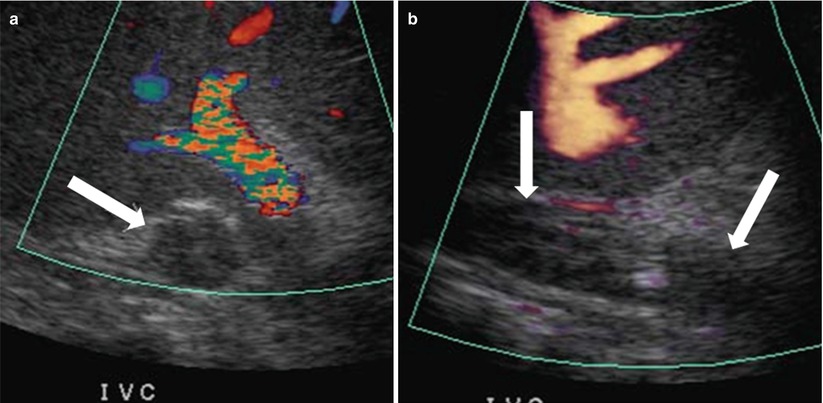
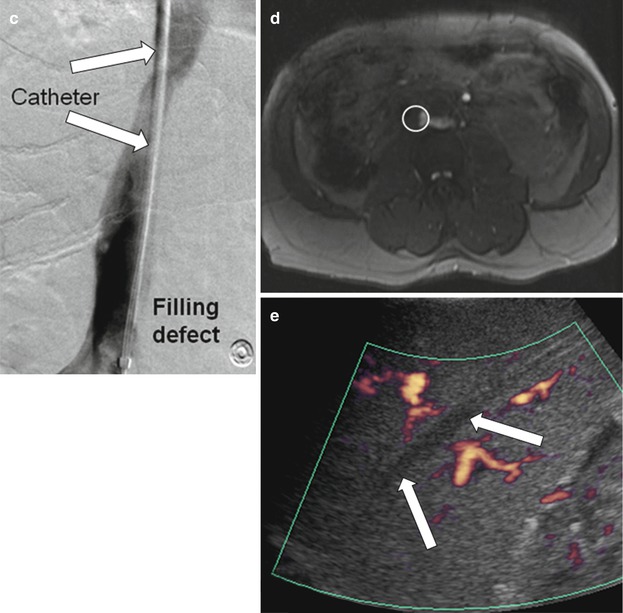
Portal vein thrombosis occurs in up to 3 % of adult liver transplant patients and 5–10 % of pediatric patients [61, 62]. On sonography, an echogenic filling defect may be seen in the portal vein, which can be confirmed by absent portal venous flow on Doppler sonography (Fig. 22.10a). Acute thrombi are anechoic [32]. Occasionally, minimal portal flow cannot be detected on Doppler sonography and requires further evaluation [2]. In these instances, CT and MRA may delineate the filling defect in the portal vein (Fig. 22.10) [63]. Rarely, transhepatic or transjugular portography is needed to achieve a definitive diagnosis [64]. Management of portal vein thrombosis may include surgical thrombectomy, resection, portosystemic shunt creation, percutaneous thrombolysis and stent placement, balloon angioplasty, or re-transplantation [65–67].
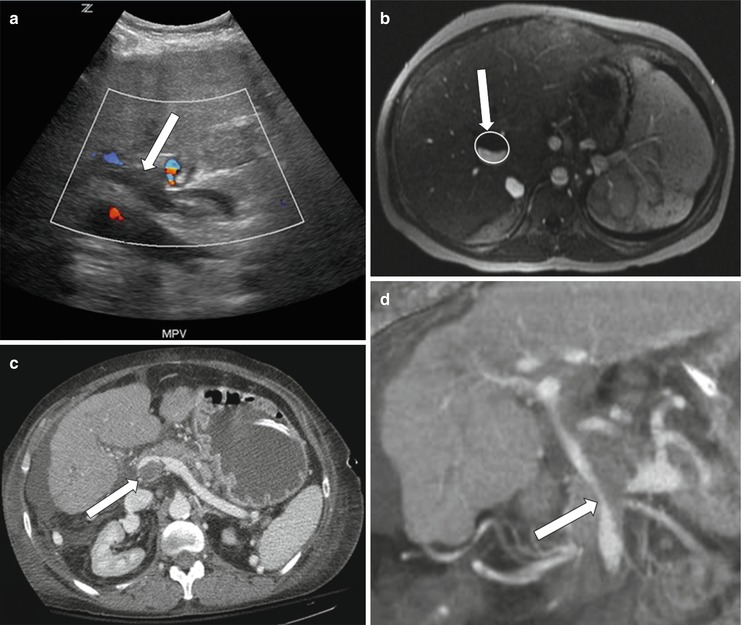

Fig. 22.10
Portal vein thrombosis. Color Doppler sonography (a) demonstrates an echogenic thrombus appreciated in the portal vein without Doppler signal (arrow). Post-gadolinium enhanced T1-weighted image (b) demonstrates low signal intensity thrombus noted in the portal vein. Axial and coronal reformatted images (c, d) demonstrate low-attenuation thrombus noted at the confluence of the splenic and inferior mesenteric veins (arrows)
Portal Vein Aneurysm
Portal vein aneurysm is an extremely rare liver transplantation complication. Only one case has been reported in the medical literature [68]. A portal vein aneurysm can be classified as intrahepatic or extrahepatic [69]. Small aneurysms are usually asymptomatic, whereas larger ones can cause duodenal compression, biliary tract obstruction, chronic portal hypertension, portal vein occlusion due to thrombosis, acute portal hypertension, and rupture [70]. On Doppler sonography, a portal vein aneurysm appears as a saccular portal vein lesion with multidirectional flow (Fig. 22.11).
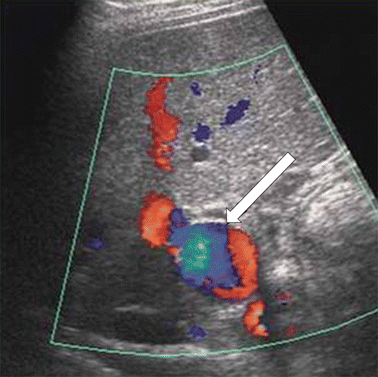

Fig. 22.11
Portal vein aneurysm. Doppler ultrasonography shows intrahepatic saccular aneurysmal dilatation of portal vein with multidirectional flow (arrow)
Key Teaching Point
Portal vein complications include stenosis, thrombosis, and aneurysm formation. In stenosis, there is an increased velocity ratio of 4:1 or a peak anastomotic velocity of more than 125 cm/s. Contrast-enhanced CT or MRI is recommended when differentiating between thrombosis and stenosis on Doppler imaging, especially if there is minimal blood flow.
IVC and Hepatic Vein Complications
IVC and hepatic vein complications include IVC stenosis, thrombosis, and rarely, torsion, as well as hepatic vein stenosis and thrombosis.
IVC or Hepatic Vein Stenosis and Thrombosis
IVC stenosis or thrombosis occurs in less than 1 % of liver transplantation patients, and hepatic venous stenosis is seen in 4–5 % [71, 72]. IVC or hepatic stenosis may present with hepatomegaly, ascites, and pleural effusions. Infrahepatic stenoses may present with lower body edema due to poor venous return [73].
IVC stenosis usually occurs at the site of surgical anastomosis. Early IVC stenosis may result from technical factors, suprahepatic caval kinking from organ rotation, or extrinsic compression from surrounding fluid collections and hematoma or secondary to graft swelling [71]. Delayed IVC stenosis may be secondary to fibrosis, a chronic thrombus, or neointimal hyperplasia. Chronic caval stenoses are more common after re-transplantation and in children [32].
In liver grafts, the normal appearance of the hepatic veins and IVC on color and pulsed Doppler is a triphasic flow pattern, reflecting the variations of blood flow with the cardiac cycle [32]. In IVC stenosis, there is a three to fourfold increase in velocity at the stenotic site compared with the pre-stenotic segment that may result in loss of hepatic vein phasic pattern or reversal of flow in hepatic veins. Grayscale sonography may reveal an echogenic thrombus with no color flow on color Doppler sonography (Fig. 22.12) [43].