IMAGING IN CARCINOMA OF UNKNOWN PRIMARY Ameya D. Puranik, Yash Jain, Richa Vaish Carcinoma of unknown primary of the head and neck (CUPHN) is defined as a scenario where the patient presents with overt cervical adenopathy but the origin/site of primary cannot be identified despite thorough medical history, clinical examination as well as standard non invasive and invasive diagnostic techniques. The reported incidence of CUPHN varies according to diagnostic workup used, with the general incidence being widely quoted as 1%–5% of all head neck cancers with an ever increasing incidence as a result of increasing viral etiology associated tumors. In the Asian countries a significant number of tumors may be Epstein–Barr virus (EBV) related and such cases should be staged according to nasopharyngeal cancers. Whereas in the western countries most of the CUPHN are human papilloma virus (HPV) related and must be classified accordingly, whenever p16 or HPV have been identified within the lymphadenopathy. Clinically, patients with CUPHN present with typical neck swelling which if confined to upper neck (level I-III) usually has primary in head and neck sub-sites. However, left level IV node (Virchow’s) node usually has infraclavicular primary disease. Nasopharynx and thyroid malignancies may metastasize to posterior triangle or level V. Hence, the onus lies more on the site of primary and extent and spread, from the therapeutic point of view. Imaging helps in not just localising the primary site, but also in providing an ideal target for tissue biopsy. A robust sample further helps in analysing the tissue with histology, immunohistochemical and molecular profiling; which can provide the exact therapeutic targets. CUPHN is essentially a diagnosis of exclusion, and what constitutes the ideal investigative protocol remains a conundrum even today within the research community. The challenge is to achieve accurate diagnosis as quickly as possible, utilizing minimum financial resources; and also causing less morbidity to the patient. Recent introduction in American Joint Cancer Commission (AJCC) and Union for International Cancer Control (UICC) eighth edition of the TNM Staging Manual has generated great promise in order to standardise the diagnostic work-up. In fact, although these lesions were previously categorized as T0 but not assigned to a specific anatomic subsite. This category is eliminated in latest AJCC except for HPV related oropharynx and EBV related nasopharynx cancer where the primary can be ascertained based on immunohistochemistry and other markers. Nowadays p16 positivity by immunohistochemistry (IHC) or EBV encoded RNA by fluorescent-in-situ hybridization (FISH) is a strong indicator of their site of origin to oropharynx (currently more than 90% of CUPHN) and nasopharynx cancers, respectively. Squamous cell carcinoma of unknown primary (SCCUP) of the head and neck is defined as clinically palpable nodes in the neck region without any evidence of the primary site on routine clinical workup which includes the history, physical examination, flexible optic endoscopy of the upper aerodigestive tract, and radiographic investigation with ultrasound, CT and/or MRI. This constitutes approximately 5% of all head and neck cancers. The pathophysiology behind why primary malignancies cannot be determined is understood; reasons which have been postulated include regression of the primary tumor tissue, small size with a slow growth rate, or histologic and/or genetic differences between the primary tumor and metastasis, and obscure anatomical locations. Nearly 80%–85% of metastatic nodes in neck region arise from tumors in the head and neck, with almost 85%–90% of the primary sites of disease detected in the oropharynx (common sites being palatine tonsils or base of tongue). Other potential primary sites of HNCUP include the vertex of the scalp, nasal vestibule, oral cavity, nasopharynx, hypopharynx, and larynx with extension into the distal esophagus (Tables 2.16.1.1 and 2.16.1.2). Courtesy: M. Albertson, et al. PET/CT evaluation of head and neck cancer of unknown primary. Semin Ultrasound CT MR 40 (5) (Oct 2019) 414–423. Jones et al. (1993) Cianchetti et al. (2009) Miller et al. (2008) Cianchetti et al. (2009) Cianchetti et al. (2009) Mendenhall et al. (1998) Rusthoven et al. (2004) Albertson et al. (2019) Imaging forms the fulcrum of diagnosis and staging in CUPHN, especially with the combination of anatomical and metabolic imaging with FDG PET/CT, detection of occult primaries and further targeting biopsies for better tissue characterisation has changed the management approach. Kwee et al. published a meta-analysis of 11 studies published between 2005 and 2007, comprising 433 patients who underwent FDG PET/CT. The primary tumor detection rate, pooled sensitivity, and specificity of FDG-PET/CT/were 37%, 84% and 84%, respectively; whereas the false positive rate (15%) was highest for oropharyngeal tumors. However, the variability of study population, timing of PET/CT studies and panendoscopy, and HPV status have a significant impact on the results. Patients with negative physical examination and office endoscopy should directly undergo FDG PET/CT. Sokoya et al studied 190 patients with CUPHN, who underwent panendoscopy after FDG PET/CT. The authors adopted this approach based on the fact that the sensitivity of PET/CT in detecting the primary site in CUPHN of squamous cell origin is 73.1% (NPV 68.9%), which implied that a significant portion of primary sites were undetected. In this cohort, PET/CT scan identified primary sites in 87 patients, who were initially designated as CUPHN, based on clinical examination studies. After panendoscopy, primary sites were identified in 32 of 103 patients (31%) with negative PET/CT scans. An added advantage of 18F FDG PET/CT is its ability to detect malignancies outside the head and neck region, such as distant metastases or synchronous or metachronous primaries at other sites. However, whole body FDG PET/CT had low specificity and a high false positive rate when it comes to primary site in tonsil, which has been extensively reported in literature. The high false-positive rate in the tonsils is due to physiologic uptake in the normal lymphoid tissue; or inflammation or tonsillitis, which is commonly seen. Other causes of false positive FDG accumulation can occur in conditions like sarcoidosis, granulomatous disease, or in benign tumors, which can hamper the specificity. False negatives can arise due to limited resolution of PET/CT scanner to detect subcentimeter sized primaries (less than 5 mm in modern PET/CT scanners). Also, uncommon histologies like mucinous, adenoid cystic or rhabdoid have low grade FDG uptake (Figs. 2.16.1.1–2.16.1.3). Extra cervical carcinoma of unknown primary commonly tends to be of adenocarcinomatous etiology although it can be of squamous cell type too. Rarely it can be of neuroendocrine origin also. It is defined as the presence of metastatic nodes, disseminated solid organ metastases and/or disseminated abdominopelvic deposits of carcinomatous cell histology (squamous, adeno or neuroendocrine) without any evidence of primary cancer on standard diagnostic workup. In only less than 30% of cases the site of primary in identified ante mortem. Post mortem examinations reveal a primary site in 60%–80% cases most often in the lung (27%), pancreas (24%) and hepatobiliary tree (8%). Current era has seen the development of tailored and specific chemotherapeutic regimens for solid tumors. Thus failure to identify the primary lesion leads to an even poorer prognosis in a cohort with already bad prognosis. Hence establishment of the site of the primary tumor is of paramount importance here to ensure timely and effective treatment and more importantly avoidance of ineffective, expensive and potentially toxic treatments. Standard diagnostic workup tends to be heterogeneous and comprises multitude of invasive and non invasive tests which can be applied. FDG PET/CT has shown promising results in this regard. It proves to be the most valuable the earlier in the course of workup it is applied as it provides pivotal evidence of the site of primary, the guide to targeted biopsy and obviates the need for further invasive diagnostic procedures. Burglin et al did a systematic review and meta-analysis of 20 studies including 1492 patients and assessed the utility of FDG PET/CT for detection of the primary tumor in adults with extra-cervical metastases from cancer of unknown primary. Although there was heterogeneity in workup and studies in general, but still FDG PET/CT had a detection rate of 40.93% and their study suggested that upfront application of FDG PET/CT does has a role in CUP as it obviates the need for further invasive diagnostic procedures. A review by Seve et al provided similar results in patients of CUP with extra-cervical metastases, FDG PET/CT revealed tumor site in 41% patients here (range 24%–63%). A large prospective randomized study is the need of the hour to confirm the potential benefit of using FDG PET/CT up front in extra cervical CUP. Majority of the retrospective studies have not compared PET/CT findings with conventional imaging. This would bring down the diagnostic yield of PET/CT for primary sites like lungs, which are diagnosed even on a chest radiograph or CT scan of thorax. As far as the data on neuroendocrine neoplasm (NEN) of unknown primary is concerned, the NCCN guidelines state that Gallium-68-labeled peptide (Ga-68 DOTA) PET/CT should be performed in cases where CT or MRI have failed to identify the site of primary. However the UK and European guidelines differ on this regard and advocate the use of Ga68 DOTA PET/CT in all cases of NEN of unknown primary and also recommend that this modality replaces the traditional somatostatin receptor scintigraphy (SRS). Naswa et al carried out a prospective study using Ga-68 DOTANOC PET/CT to localize primary tumor in 72 patients and found that it had sensitivity of 78%. Another prospective study was conducted by Prasad et al in 59 patients with NEN of unknown primary, Ga68 DOTANOC PET/CT was able to localize the primary in 59% of patients. The main diagnostic challenge for PET/CT in CUP lies in minimizing the number of false negatives results. It is also well known that FDG is not a specific marker of malignancies since uptake is seen in inflammatory tissue also. To overcome these drawbacks PET tracers reflecting more specific biologic tumor characteristics are under development and clinical investigation. Since the receptor expression on different tumor types has been identified in recent times, the choice of investigation for detection of primary sites has become more focused. Advanced immunohistochemistry and molecular markers provide us with valuable clues to which metabolic pathway to focus on, and we now have molecular imaging radiotracers targeting specific receptors, illustrated in Table 2.16.1.3. These receptors also provide suitable targets for planning radionuclide therapy, based on the principle of Theranostics. Management of CUP is challenging due to its clinical presentation late in the course of disease, difficulty in selecting the modality of choice for diagnosis and natural resistance to standard treatment regimes, as compared to other malignancies. Hence, there is immense onus on the choice of imaging and furthermore, the crux of imaging in CUP lies in early detection to reveal a potentially curable cancer that would be fatal if left untreated. FDG PET/CT by virtue of its ability of whole body imaging has been used for decades to screen underlying malignancies in asymptomatic individuals. Over the years FDG PET/CT has emerged as an excellent alternative to conventional imaging by CT or MRI in detection of unknown primary. It is not only useful for detection of primary tumor sites but also for evaluating the extent of disease, possible pattern of spread and selecting the most appropriate site for biopsy. Overall PET/CT with a wide array of targeted radiotracers is a crucial tool in the diagnostic armamentarium in patients with cervical and extra cervical CUP and must be considered early in the diagnostic algorithm. Eg. PSMA PET/CT for suspected prostate cancer. LYMPH NODE METASTASIS Abhishek Mahajan, Vatsal Kania, Anurag Gupta, Ankur Chand, Pooja Pande Lymphogenous metastasis means penetration of tumor cells into the lymphatic vessel and then by lymph flow to nearby or distant lymph nodes. It is a significant prognostic factor for most of the malignancies. The location and the number of metastatic LN (lymph node) have a direct impact on the staging of the tumors, and therefore, affect the selection of a treatment plan and patient’s survival rate. However, over-staging often leads to unnecessary extended surgical interventions and added morbidity. On the other hand, under-staging may lead to an increase in recurrence rate and may reduce the survival time. The staging is determined by the clinical and pathologic status of the regional lymph node (RLNs). The clinical diagnosis is heavily dependent upon clinical and radiological observations but the gold standard that provides the most important information for staging is the histological evaluation of the biopsied node(s). The dangerous part of any cancer is its potential to spread or metastasize. Metastasis is a multiplex process involving the detachment of cells from the cancerous tissue, the regulation of cell motility and invasion, and the proliferation and evasion through the lymphatic system or blood vessels (Figs. 2.16.2.1 and 2.16.2.2). The spread of malignancy to nearby lymph nodes is called local or regional metastasis and the spread of the tumor to a distant organ is called distant metastasis. Usually, the first lymph node draining the cancer is involved and is referred to as the sentinel node. The concept behind how tumor cells migrate to LNs is still evolving. There are different theories regarding the involvement of regional lymph nodes in metastasis, however in general illustrated below (Fig. 2.16.2.3). Routinely, all the palpable cervical lymph nodes are considered positive for regional metastasis in oral cancer. The site, number, shape, size, tenderness, consistency, and fixity to underlying structures are the criteria commonly used during a clinical examination of the cervical lymph nodes. Even though palpation has the advantage of being easy and inexpensive to perform and repeat, it is generally accepted to be inaccurate. Therefore, appropriate investigations should be carried out which have high accuracy for the N0 neck. Conventional methods such as ultrasound (USG), computed tomography (CT), magnetic resonance imaging (MRI) which provides morphological and anatomical assessment are commonly used and some novel methods like diffusion-weighted imaging of MRI, positron emission tomography (PET), or PET/CT and MRI are sometimes added for staging (Fig. 2.16.2.4). An ideal imaging method should distinctly detect and exhibit the location and structural characteristics of LNs, accurately distinguish the malignant nodes from benign ones. It should be commonly available, affordable, easy to interpret, non-invasive, and non-radiative. Two main criteria useful for the evaluation of lymph nodes are size and morphologic criteria (Fig. 2.16.2.4). Also, it is essential to mention extracapsular extension and involvement of vessels/adjacent organs which might change management. Grayscale USG is the commonest method for evaluating superficial LNs. High-frequency linear array USG transducers are typically used for evaluating superficial LNs (i.e., neck, inguinal region, or the axillary fossa). It evaluates the nodes for Size and morphologic criteria (Table 2.16.2.1). The absence of a fatty hilum and cortical hypertrophy in an LN is regarded as the most specific predictive factor for metastasis. Moghaddam et al. noted an echogenic hilum of cervical LN in 81% of benign LNs and 55% of malignant ones, thus spotting the absence of hilum as a marker for LN involvement in USG. Besides hilum, some other specific signs within LNs can be displayed by the USG, such as necrosis and microcalcifications. Color Doppler is useful in distinguishing non-metastatic nodes from the metastatic node based on the vascularity of lymph nodes (Table 2.16.2.1). CT has now become the most important and routinely used modality for pretreatment staging and evaluation of cancer patients. As a structural imaging method, the CT diagnostic criteria for metastatic LNs mainly depend on location, size, and morphologic criteria (Table 2.16.2.1 and Fig. 2.16.2.5). Among them, size is still the most common criterion with a wide variable range from 5 to 15 mm. A short-axis diameter of more than 1 cm is usually accepted as a threshold for malignancy in most studies. However, the diagnostic sensitivity of this standard is obviously weakened by a high false-negative ratio, viz. 16%–74% metastatic LNs with normal size. Hence, it is difficult to choose an appropriate cutoff to balance the sensitivity and specificity at the same time. Conventional MRI has high repeatability and reliability in assessing LNs status; however, it is not superior to USG and MDCT in diagnostic accuracy. In a study about LN detection of pelvic malignancies, Sarkar et al. reported that MRI detected more LNs compared to CT in all nodal stations, like the external iliac, obturator, and internal iliac chains. Based on the size, the numbers of nodes detected by CT and MRI were equal when nodal size is more than 10 mm; however, when size is between 1 and 5 mm, MRI is capable of detecting more nodes. As a simple, non-invasive, and non-contrast tool, diffusion-weighted imaging (DWI) has become the most potential MRI sequence in LNs mapping in recent years. Furthermore, fused with high-resolution T2-weighted images can overcome the weakness of DWI on anatomic details and result in its increased performance. Regional nodal metastasis is the single most important prognostic factor for HNSCC, and evaluation of cervical nodes is an essential component of staging and treatment planning. Knowledge of cervical anatomy and nodal classification is essential to accurately describe cervical nodal metastases in HNSCC. The American Joint Committee on Cancer (AJCC) has classified neck lymph nodes from levels I through VII (Table 2.16.2.2, Figs. 2.16.2.6 and 2.16.2.7). IA – submental, IB – submandibular Anterior belly digastric muscle. Posterior margin submandibular gland Anterior oral cavity, lip, sinonasal Anterior cervical/upper jugular IIA – posterior margin submandibular gland and posterior margin IJV IIB – posterior margin sternocleidomastoid Oropharynx, posterior oral cavity, supraglottic larynx, parotid gland Middle jugular Inferior margin hyoid Glottic, subglottic, hypopharyngeal region Lower jugular Inferior margin cricoid Subglottis, thyroid, cervical esophagus Posterior compartment or spinal accessory Posterior margin of sternocleidomastoid Nasopharynx, skin carcinoma of neck or occipital region Visceral or central compartment Medial margin of carotid artery, inferior margin of hyoid and superior aspect of manubrium Subglottic, thyroid and cervical esophagus Superior mediastinal Retropharyngeal Superior aspect of manubrium and innominate vein. Medial margin of internal carotid artery to level of hyoid Subglottic, thyroid and cervical esophagus Nasopharynx, sinonasal Neck node staging under the TNM staging for head and neck tumors according to AJCC seventh edition as shown in Table 2.16.2.3 and newer changes are illustrated in Table 2.16.2.4. This staging system excludes the nasopharynx (Table 2.16.2.5) and thyroid (Table 2.16.2.6). Note: * Clinical or radiological Extranodal extension (ENE) should be recorded as E− or E+. Note: Regional Lymph nodes are the central compartment, Lateral cervical and upper mediastinal lymph nodes. The approach to cervical adenopathy is illustrated in Figs. 2.16.2.8 and 2.16.2.9 below. Thoracic lymph nodes are separated into two types: Diaphragmatic lymph nodes – situated on the thoracic surface of the diaphragm as summarized in Table 2.16.2.7. Functional division – This division is based upon drainage of lymph within the thorax. International Thymic Malignancy Interest Group (ITMIG) has divided the mediastinum into three functional divisions: pre-vascular, visceral, and paravertebral mediastinum. Nodes in these regions, therefore, help in localizing lesions, thereby helping to narrow down the lengthy differential diagnoses. Structural division/Nodal station – The concept of structural staging was introduced to make accurate staging and mapping of the disease and plan procedures accordingly. The International Association for the Study of Lung Cancer (IASLC) Lymph Node Map defined the lymph node stations in the hila and mediastinum (Table 2.16.2.8 and Fig. 2.16.2.12) for the staging of lung cancer; however, these stations can be applied to tumors of the breast, esophagus, and pleura and to lymphomas. Lymph Node Zones and Stations As per IASLC, the thoracic lymph nodes are divided into 14 stations, which are grouped into seven zones. Mediastinal pleura contains stations one to nine, while visceral pleura contains stations 10–14. Stations 1, 2, 4, and 10–14 additionally have and L and R designators for left and right, while station 3 has P and A designators for posterior and anterior. The midline of the trachea is used to designate which lymph nodes are 1R and 1L; whereas the left lateral border of the trachea is used to differentiate between 2R and 2L and 4R and 4L nodes. However, additional lymph node regions (like internal mammary nodes) in the thorax that are not included in this nomenclature can also be involved by tumor metastases. The thoracic duct is an important component of the thoracic lymphatic system in addition to mediastinal lymph nodes; beginning at the superior aspect of the cisterna chyli (at the level of the L2 vertebra), courses cranially to the region of the T5 vertebra, where it drains into the junction of the internal jugular vein and left subclavian vein. It drains 75% of the body’s lymph fluid into the venous system, except for the right portion of the head and right arm and (which finally drain into the junction of the right internal jugular and subclavian veins). Various cancers like lung, breast, lymphoma, esophageal, and malignant mesothelioma have different lymphatic drainage pathways in the thorax that are useful in the staging. Computed tomography (CT) is the primary modality for imaging malignancy in the chest for staging, sampling, planning treatment, evaluating the response, and estimating prognosis. The majority of CT studies record the short-axis diameter (SAD) of a lymph node, as this is the most reproducible measurement and are generally considered enlarged in more than 10 mm SAD; while hilar and periesophageal nodes can be considered enlarged beyond 7 mm SAD and diaphragmatic nodes beyond 5 mm in short-axis diameter. No size criteria are available for retrocrural, internal mammary, and other parietal nodes, and hence, detection of these nodes should be considered abnormal. Nodal size cannot be used reliably to indicate metastatic involvement. In patients with lung cancer, about 13% of nodes measuring less than 1 cm were metastatic while one-third of nodes 2–4 cm in diameter were hyperplastic and did not contain metastases as per McLoud et al. Here, FDG PET has an increasing role in the detection of diseased lymph nodes that appear normal at CT alone; pathologic confirmation of mediastinal nodal involvement should be undertaken when there is increased uptake in mediastinal lymph nodes. The lung’s lymphatic supply comprises the parenchymal and pleural network. The parenchymal lymphatics are situated in the interlobular septa and broncho vascular bundles which drain sequentially into the intralobular, interlobular, lobar, and finally, the hilar nodes and the hilar nodes drain into the mediastinal nodes (Figs. 2.16.2.13 and 2.16.2.14) whereas the pleural lymphatics drain into hilar node while anastomosing with the parenchymal lymphatics. Most lung parenchymal tumors drain to the hilar nodes before reaching the mediastinum. However, mediastinal metastases may occur by directly bypassing the hilar nodes. Direct connection may exist with the thoracic duct, enabling systemic nodal metastases without mediastinal node involvement. Table 2.16.2.9 illustrates predilection for regional node involvement as per lobar tumor. In a patient with lung cancer, the nodal status determines surgical resectability. AJCC eighth edition has classified the nodal staging as illustrated in Table 2.16.2.10. They are divided into Hodgkin and non-Hodgkin disease. There is frequent involvement of the anterior mediastinal and paratracheal regions and a tendency for contiguous spread to subcarinal, peridiaphragmatic, periesophageal, and internal mammary nodes nodal groups in decreasing order of frequency (Fig. 2.16.2.15). In most patients, two or more nodal groups are involved at the initial presentation. Involvement of the posterior mediastinal lymph nodes is associated with pleural, retrocrural, and retroperitoneal disease. Isolated hilar involvement is rare and should suggest an alternative diagnosis. Lymph node involvement is demonstrated by enlargement of a node detected clinically or by imaging when alternative pathology may reasonably be ruled out. Imaging criteria include demonstration of fluorodeoxyglucose (FDG) avidity on FDG positron emission tomography (FDG-PET) or unexplained node enlargement on CT. Suspicious nodes should always be biopsied if treatment decisions are based on their involvement. preferably with an excisional biopsy; fine needle aspirations are strongly discouraged because of the potential for false negatives or misdiagnosis because of loss of lymph node architecture. Core needle biopsy may be able to provide adequate material for diagnosis, particularly of a secondary site. Non-Hodgkin lymphoma is a more heterogeneous group of diseases. However, It is difficult to differentiate Hodgkin disease from non-Hodgkin lymphoma on the basis of nodal distribution alone. High-grade tumor and large bulky mediastinal adenopathy (greater than 10 cm in transverse diameter or greater than one-third of the thoracic diameter) is associated with an increased risk of relapse and therefore, requires both chemotherapy and radiation therapy. An accurate description of the extent of the disease is important for radiation therapy planning. After treatment, diseased lymph nodes may show irregular or eggshell calcifications at CT. Recurrent disease is common in the pericardial and internal mammary lymph nodes, as these are usually not included in the radiation field. Lymph node calcification before treatment is unusual. The esophagus is divided into cervical and thoracic portions each having its separate nodal drainage. The lymphatics of the upper two-thirds of the esophagus drain the cervical and mediastinal nodes. The lymphatics of the lower one-third drain toward the abdomen as illustrated in Table 2.16.2.11 and Fig. 2.16.2.17. The N stage of the disease is determined by the number of regional LNs involved by the tumor as shown in Table 2.16.2.12 and Fig. 2.16.2.16. Also, not all nodal stations used by the IASLC for lung cancer are considered regional LNs for esophageal cancer staging. Endoluminal ultrasound has improved accuracy in the assessment of local lymph node metastases when compared with CT (80% compared with 51%). They are aggressive neoplasms related to asbestos exposure, arising from mesothelium lining pleura, pericardium, and peritoneum with nodal staging as described in Table 2.16.2.13. The anterior pleural and diaphragm lymphatics in the upper and middle thorax drain into the internal mammary lymph nodes and while in the lower thorax they drain into peridiaphragmatic lymph nodes. The posterior pleural lymphatics drain into the extrapleural lymph nodes, which lie in the paraspinal fat. The posterior diaphragm lymphatics drain into the posterior mediastinal nodes, paraaortic lymph nodes, nodes along the celiac axis, and gastrohepatic ligament. The regional lymph nodes (Fig. 2.16.2.18) include the following:
2.16: Imaging of metastasis
Introduction
CUPHN: Current status
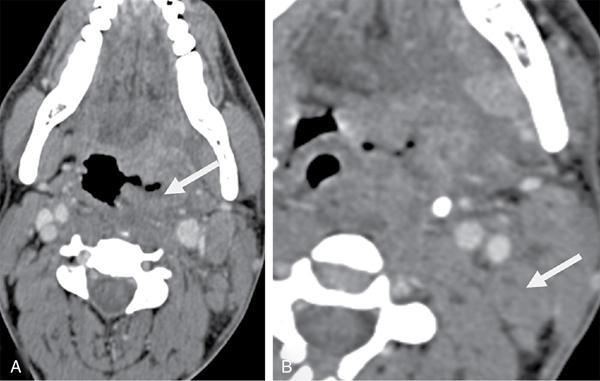
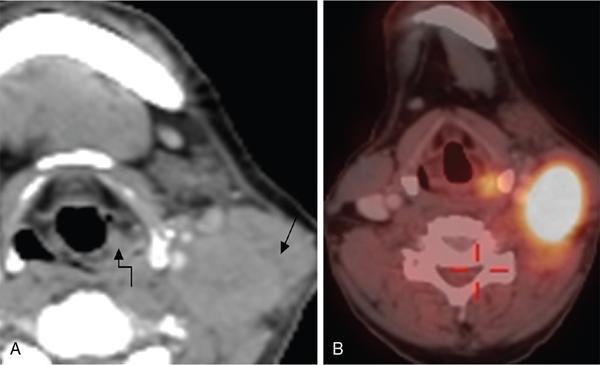
Unknown primary in head and neck of squamous cell origin
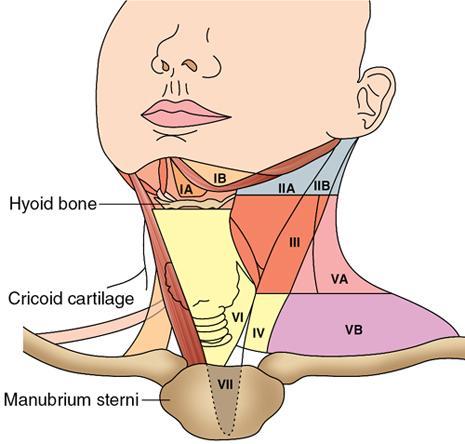
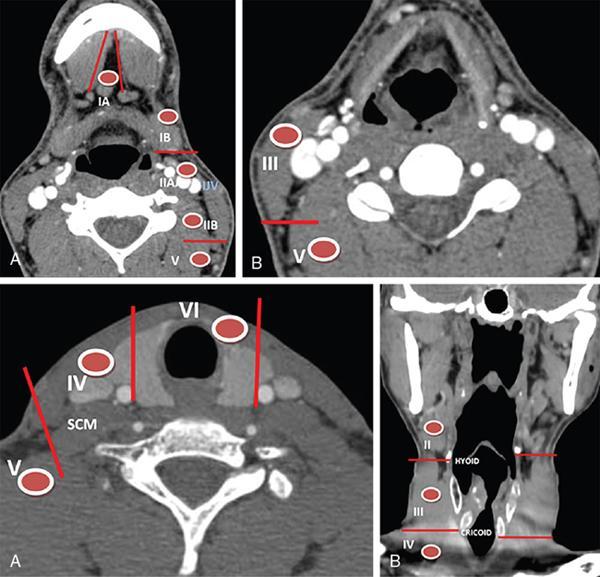
Cervical Nodal Levels
Sites of Primary
Level IA
Oral cavity, face
Level IB
Oral cavity, nasal cavity, face
Level II
Oral cavity, nasal cavity, pharynx, larynx, oropharynx
Level III
Oropharynx, pharynx, larynx, hypopharynx
Level IV
Oropharynx, larynx, hypopharynx, below clavicles
Level V
Nasopharynx, thyroid, scalp
Level VI
Thyroid, larynx, hypopharynx, below clavicles
Intra-parotid
Parotid gland, lateral face, scalp
Supraclavicular
Thyroid, non-head and neck primary
Diagnostic Technique
Yield
Reference
Panendoscopy (following negative physical examination and office endoscopy)
40%–55%
Panendoscopy (following negative conventional imaging and PET/CT
35%
Computed tomography (following negative physical examination)
62%
FDG PET/CT (following negative panendoscopy and CT imaging)
17%–29%
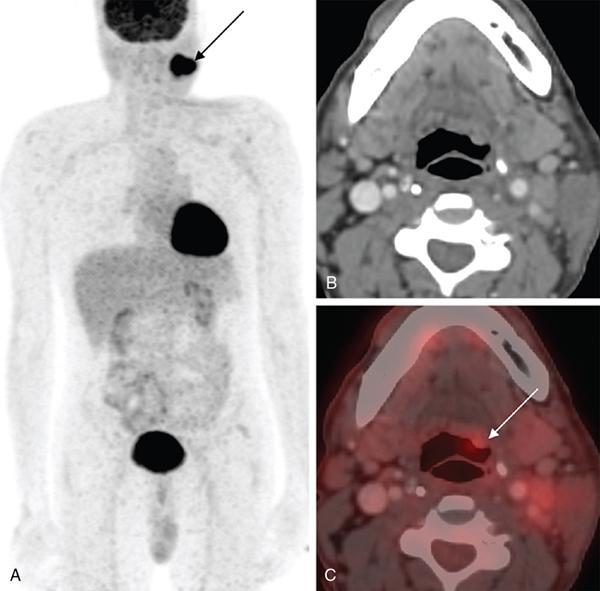
FDG PET/CT in CUPHN
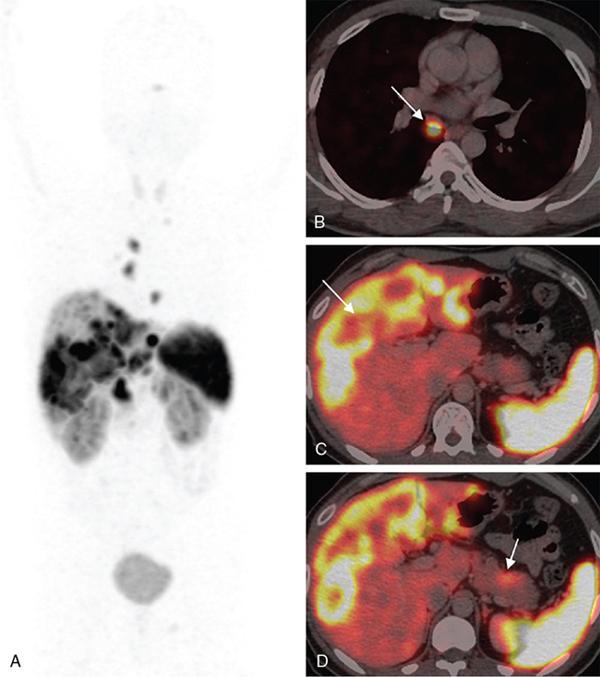
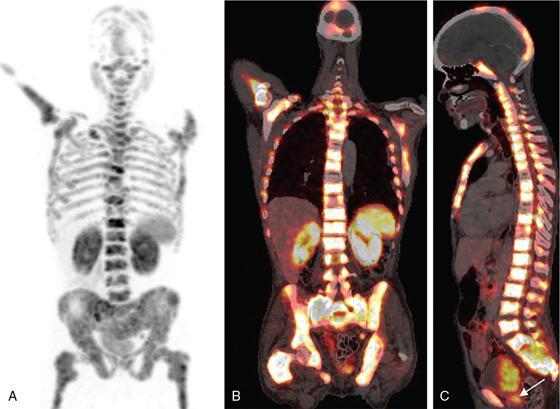
Extracervical CUP
PET/CT for extracervical CUP
Future directions
Receptor Expression
Primary Malignancy
Radiotracer (Pet/Ct)
PSMA (prostate specific membrane antigen)
Prostate cancer
Ga-68-PSMA
Angiogenesis pathway
Renal cell carcinoma
Ga-68-RGD (arginine-glycine-aspartic acid)-
Sodium-iodide symporter
Thyroid cancer
18F-tetra-fluoro-borate (TFB)
Breast cancer
Glucagon-like peptide-1 (GLP-1)
Pancreatic beta cell tumors, e.g. Nesidioblastomas
Ga-68-DOTA-Exendin
Amino acid transporters
Glial tumors
18F-flouro-ethyl-tyrosine (18F-FET)
Fibroblast-activation-protein
Adenocarcinomas
Ga-68-FAPI (fibroblast activation protein inhibitor)
Conclusion
Teaching points
Introduction
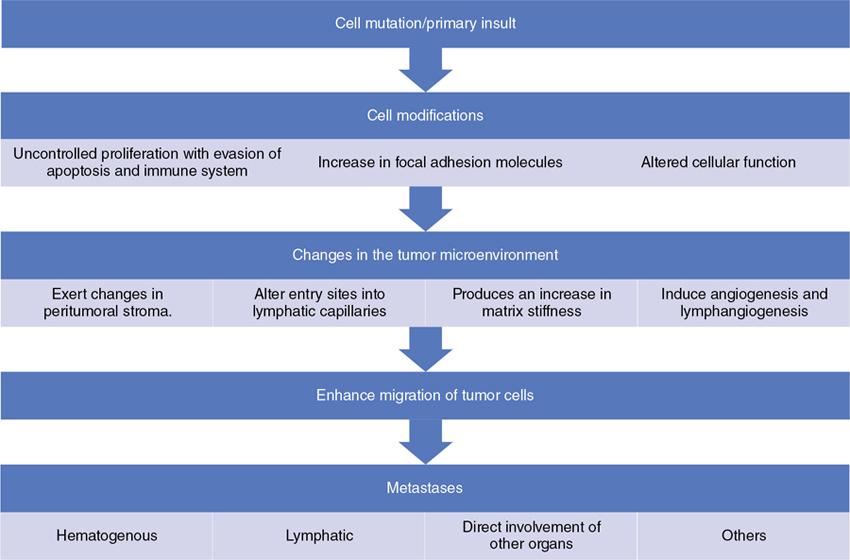
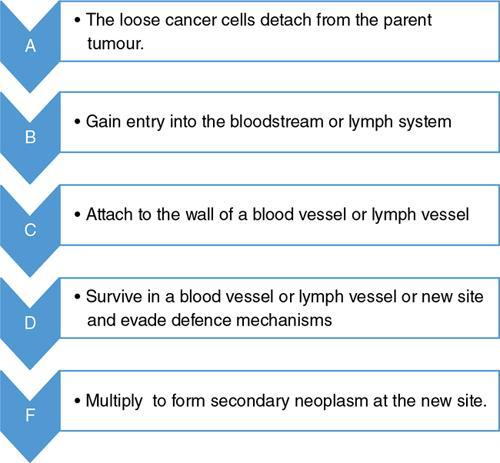
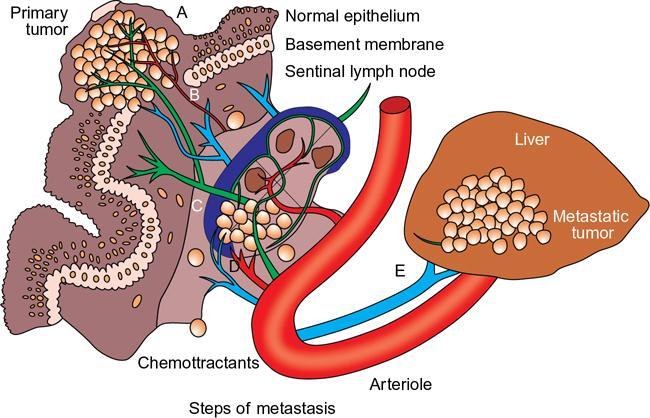
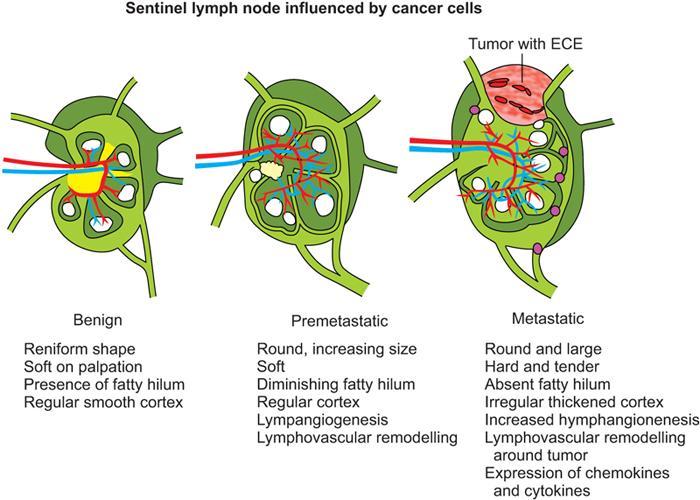
Mechanisms—how tumors metastasize to lymph nodes
Clinical aspect of lymph node metastasis
Clinical diagnosis overview
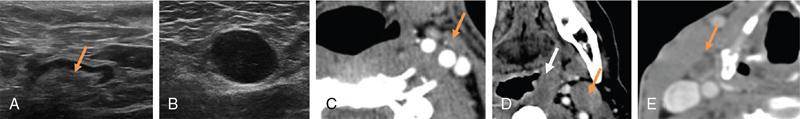
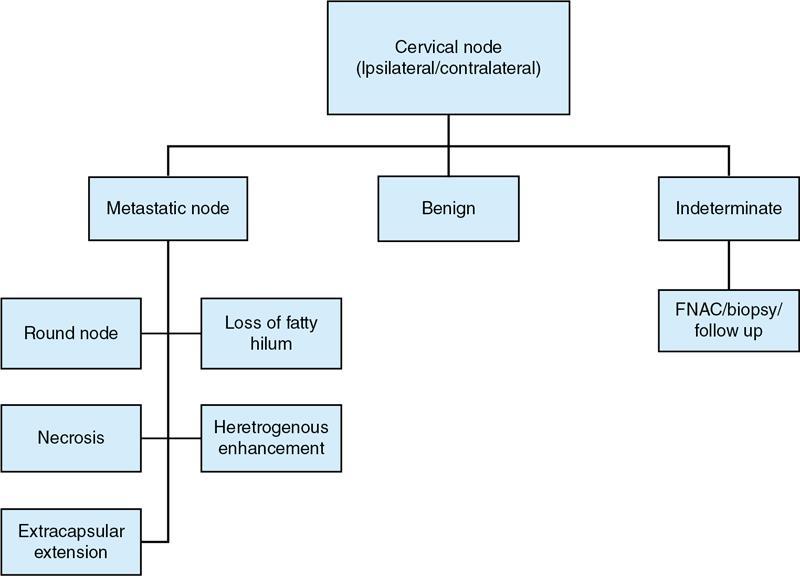
Radiological imaging
Grayscale USG
Radiological Characteristics
Benign
Metastatic
Distribution
Scattered
Clustered
Size criteria (short diameter)
Neck <1.0 or <1.5 cm
Neck >1.0 or >1.5 cm; chest >1.0 cm; abdominal and pelvic >1.0 or 0.8 cm
Chest <1.0 cm; abdominal and pelvic <1.0 or 0.8 cm
L/S ratio (long axis/short axis diameter)
≥1.5
<1.5
MORPHOLOGIC CRITERIA
Shape
Oblong
Round
Texture
Homogenous
Heterogenous
Margin
Regular
Irregular
Capsule intactness
Capsule intact
Extracapsular extension (ECE)
Internal structures
Intact fatty hilum commonly
Absence of fatty hilum commonly
Fatty hilum
Necrosis
Almost without
Commonly but not always
Calcification
Coarse
Micro
DOPPLER
Flow pattern
Central
Peripheral and mixed
RI value
<0.8
>0.8
PI value
<1.6
>1.6
ENHANCED CT
Uniformity of enhancement
Homogenous
Ring enhancement
Enhanced pattern
Moderate
Obvious or moderate
Color doppler
Computed tomography (CT)
Magnetic resonance imaging (MRI)
Conventional MRI
Diffusion-weighted imaging
Head and neck malignancy overview
Level
Anatomical Name
Key Landmarks
Primary Drainage Site
I
II
III
IV
V
VI
VII
Role of neck imaging in the staging of HNSCC
Head and neck cancer nodal staging (N)
Nx
Regional lymph nodes cannot be assessed
N0
No regional node metastasis
N1
Metastasis in single ipsilateral node, ≤3 cm in greatest dimension
N2a
Metastasis in single ipsilateral node more than 3 cm but not more than 6 cm in greatest dimension.
N2b
Metastasis in multiple ipsilateral node, none more than 6 cm in greatest dimension.
N2c
Metastasis in bilateral or contralateral lymph nodes, none more than 6 cm.
N3
Metastasis in a lymph node more than 6 cm in greatest dimension.
1.
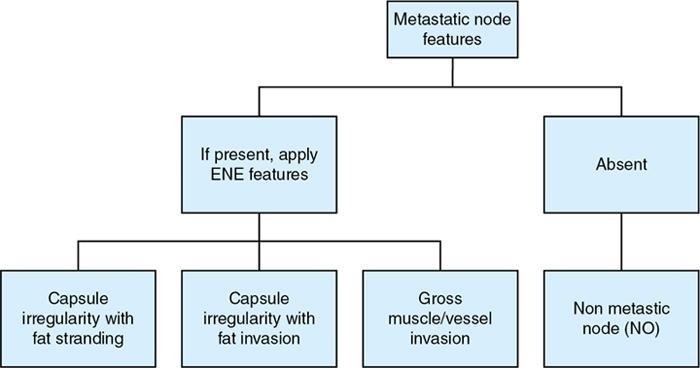
It includes addition of extranodal extension (ENE) to N stage in all head and neck cancer except HPV positive oropharyngeal cancer
2.
Modification of N3 staging
N3a Same as N3 before, but ENE –ve i.e., Metastasis in a lymph >6 cm.
N3b Single ipsilateral node >3 cm in greatest dimension and ENE positive; or multiple ipsilateral, contralateral or bilateral lymph node, any with ENE.
Nx
Regional lymph nodes cannot be assessed
N0
No regional lymph node metastasis
N1
Unilateral metastasis in cervical lymph nodes, 6 cm or less in greatest dimension, above supraclavicular fossa and/or unilateral or bilateral retropharyngeal lymph nodes, 6 cm or less in greatest dimension.
N2
Bilateral metastasis in cervical lymph nodes, 6 cm or less in greatest dimension above supraclavicular fossa.
N3
Metastasis in lymph node >6 cm and/or to supraclavicular fossa
N3a
Greater than 6 cm
N3b
Extension to the supraclavicular fossa
Nx
Regional lymph nodes cannot be assessed
N0
No regional lymph node metastasis
N1
Regional lymph node metastasis
N1a
Metastasis to level VI (pretracheal, paratracheal, and prelaryngeal/Delphian lymph nodes).
N1b
Metastasis to unilateral, bilateral or contralateral cervical levels (I, II, III, IV or V) or superior mediastinal lymph nodes (level VII)
Radiological features for assessing metastatic nodes
Thoracic and axillary lymph nodes and their metastases
Site
Drainage
Drains to
Anterior group
Base of the xiphoid process
Convex hepatic surface, anterior lymph vessels from the diaphragm, parasternal nodes
Parasternal nodes
Lateral group
Point where the phrenic nerves enter the diaphragm, lie within the fibrous pericardium anterior to the intra-thoracic end of the inferior vena cava
Central diaphragm, the convex surface of the liver
Posterior mediastinal, parasternal and brachiocephalic nodes
Posterior group
Posterior aspect of the crura (retrocrural space)
Lateral aortic nodes
Posterior mediastinal nodes
Mediastinal nodes
Supraclavicular zone (station 1)
From the lower margin of the cricoid to the clavicles and the upper border of the manubrium includes LNs in the sternal notch, supraclavicular, and lower cervical regions (overlaps with cervical LN level IV, V, VI used in head and neck cancers).
UPPER ZONE (SUPERIOR MEDIASTINAL LNs)
Station 2R. Upper paratracheal
From upper border of manubrium to the intersection of caudal margin of innominate (left brachiocephalic) vein with the trachea.
Station 2L. Upper paratracheal
From the upper border of manubrium to the superior border of aortic arch
Station 3A. Pre-vascular
Located anterior to the vessels (superior vena cava and left carotid artery) in prevascular space; behind the sternum; extending from the apex of the chest to the carina
Station 3P. Pre-vertebral
Behind the esophagus in visceral space; located in the area posterior to the trachea, extending from the apex of the chest to the carina
Station 4R. Lower paratracheal
From the inferior margin of left brachiocephalic vein with the trachea to the lower border of the azygos vein.
Station 4L. Lower paratracheal
From upper margin of the aortic arch to the upper rim of the left main pulmonary artery.
AORTOPULMONARY ZONE
Station 5. Subaortic
In aortopulmonary window; lateral to the ligamentum arteriosum. The lower margin of the aortic arch serves as the upper border of station 5, while the superior margin of the left pulmonary artery demarcates the lower extension.
Station 6. Para-aortic
Para-aortic LNs on anterior and lateral aspect of the ascending aorta and aortic arch. The phrenic nerve may be used as a landmark for identifying lymph nodes that are classified as paraaortic.
LOWER ZONE (INFERIOR MEDIASTINAL LNs)
Station 7. Subcarinal
Below the carina and between the mainstem bronchi. The distal aspect of the bronchus intermedius and origin of the left lower lobe bronchus is used to demarcate the right and left inferior extensions of station 7.
Station 8. Paraesophageal nodes below carina.
Found inferior to the distal aspect of the bronchus intermedius to the esophageal opening of the diaphragm
Station 9. Pulmonary ligament
Associated with the pulmonary ligaments. These “ligaments” are not ligaments but represent the mediastinal parietal pleural reflections that occur below the right and left pulmonary roots (9R and 9L).
HILAR ZONE + INTERLOBAR AND PERIPHERAL ZONE (EXTRA-MEDIASTINAL LNs)
Station 10. Hilar nodes
Along the right and left mainstem bronchi, before they bifurcate
Station 11. interlobar nodes
located between the lobar bronchi, just beyond the bifurcation of each mainstem bronchi.
Stations 12–14. Peripheral node (lobar, segmental, and subsegmental lymph nodes)
These LNs are infrequently seen and difficult to accurately categorize on imaging; hence many use the broad term of peripheral LNs for stations 12–14.
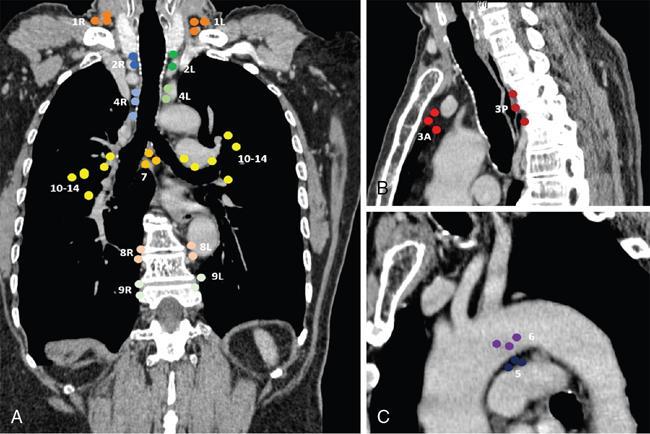
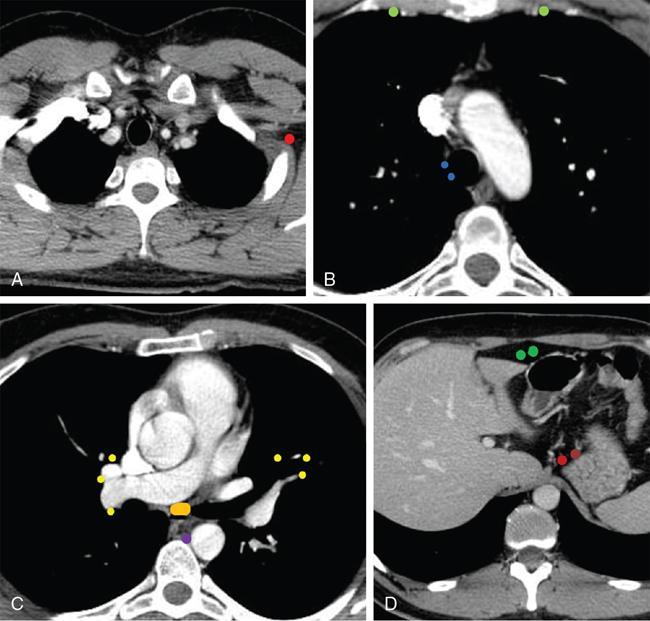
Clinical significance
Patterns of lymphadenopathy in thoracic malignancies
Size criteria
Limitations of size criteria
Lung cancer
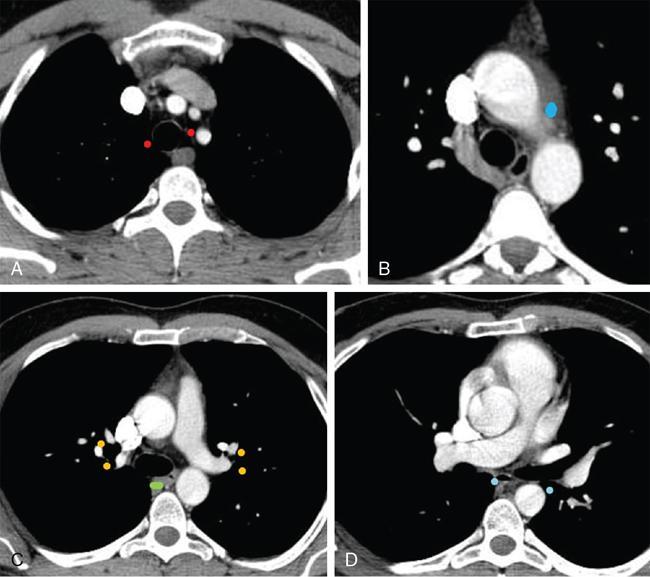
Lobe
Nodal Stations
Right upper lobe
The right paratracheal and anterior mediastinal nodes
Right middle and lower lobe tumors
Subcarinal nodes and subsequently into the right paratracheal and anterior mediastinal nodes
Left upper lobe tumors
Subaortic and paraaortic nodes
Left lower lobe tumors
Subcarinal and subaortic nodes
Nodal Staging
Nodal Stations
Management
N0
No regional lymph node metastases
Surgery in the absence of mediastinal invasion by tumor, malignant pleural effusion, satellite nodules, or metastases
N1 (within the confines of the pleural reflection)
Metastatic ipsilateral hilar/peri bronchial/intrapulmonary node
Surgery in the absence of mediastinal invasion by tumor, a malignant pleural effusion, satellite nodules, or metastases
N2 (outside the pleural reflection)
Metastases to ipsilateral mediastinal and/or subcarinal node
These cases may be amenable to surgery, but treatment also involves chemotherapy and irradiation.
N3 (Advanced disease)
Contralateral mediastinal or hilar node involvement/uni or bilateral supraclavicular node
Non-surgical candidates
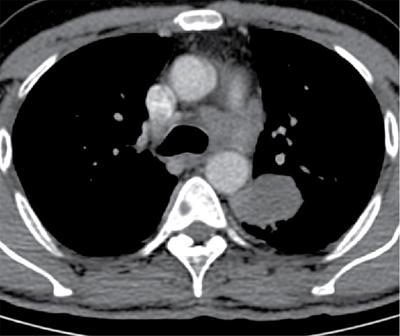
Lymphoma
Therapy planning in lymphoma
Esophageal carcinoma
Cervical esophagus
Supraclavicular, internal jugular, upper and lower cervical, and periesophageal nodes
Thoracic esophagus
Paratracheal, periesophageal, and subcarinal nodes
Gastroesophageal junction tumors
Nodes adjacent to the diaphragm, pericardium, left gastric artery (gastrohepatic ligament), and celiac artery
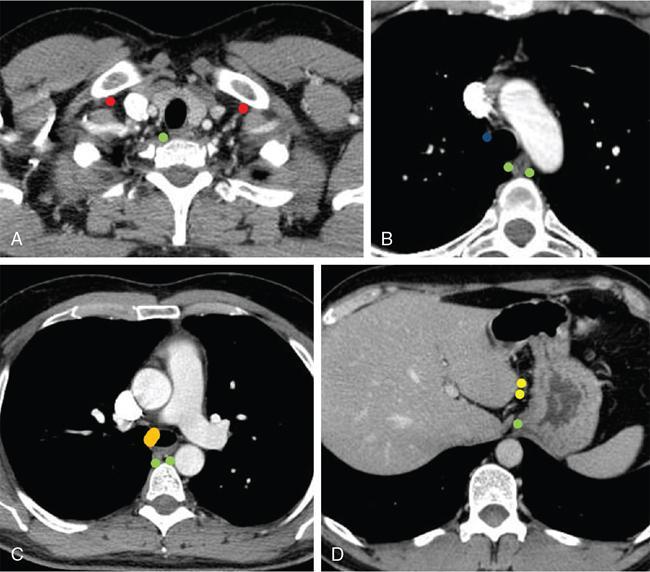
N0
No regional metastases
N1
Regional lymph node (1–2) metastases
N2
Regional lymph node (3–6) metastases
N3
Regional lymph node (≥7) metastases
M1a
Upper esophagus- metastases to cervical nodes
Lower esophagus- metastases to celiac nodes
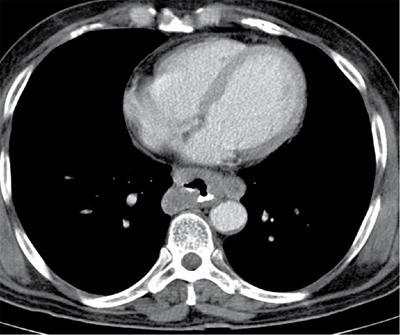
Malignant pleural mesothelioma
N0
No regional metastases
N1
Metastases to ipsilateral peribronchial, hilar nodes or mediastinal nodes(including internal mammary nodes, peridiaphragmatic, pericardial fat pad or intercostal lymph nodes)
N2
Metastases to contralateral mediastinal, internal mammary or hilar nodes and/or ipsilateral or contralateral supraclavicular node
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree