Algorithm 24.1 Benefits of Pancreas Transplantation
Both pancreas allograft and patient survival have improved considerably over the past 20 years [1]. The 1-year patient survival rate is now approximately 95 %, while 5-year survival approaches 83 % [1]. SPK transplants result in the best pancreas allograft survival, with 86 % functioning at 1 year following surgery [1]. While PTA has the worst 1-year pancreas allograft survival, up to 78 % still function at 1 year following surgery [1]. Improved pancreas allograft and patient survival rates are likely due to a variety of causes, including improved surgical techniques, refined immunosuppressive medical therapy, the development of dedicated transplant centers, and improved donor and recipient selection criteria [4, 9]. Most pancreas transplantation procedures that fail in the early postoperative setting are the result of either technical causes (such as thrombosis, bleeding, anastomotic leaks, and infection) or acute rejection, while allografts that survive beyond 6 months most commonly fail due to chronic rejection [10, 11].
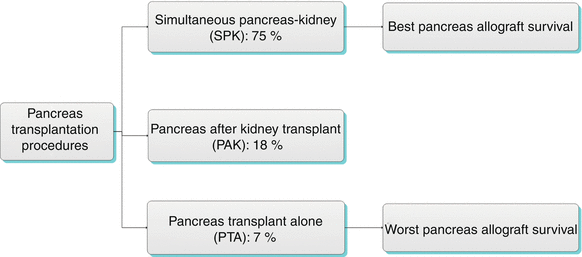

Algorithm 24.2 Types of Pancreas Transplantation Procedures
Surgical Techniques and Transplant Anatomy
The deceased donor (cadaveric) pancreas allograft is harvested along with a portion of the donor duodenum. Prior to allograft placement in the recipient, an arterial “Y-graft” is constructed from the donor’s common iliac, internal iliac, and external iliac arteries. The external iliac portion of the Y-graft is anastomosed to the donor superior mesenteric artery, while the internal iliac portion of the Y-graft is anastomosed to the donor splenic artery (Fig. 24.1).
Deceased donor whole pancreas allografts are usually placed in the recipient’s peritoneal cavity using a pelvic midline approach [12, 13]. The common iliac artery portion of the Y-graft is anastomosed to the recipient’s common or external iliac artery in an end-to-side fashion [12]. The donor portal vein is most commonly anastomosed to a systemic vein (the common iliac vein, external iliac vein, or IVC). Less commonly, the donor portal vein is anastomosed to the recipient’s portal venous system, even though this technique has the advantage of avoiding undesired systemic circulation hyperinsulinemia and potential associated complications [12].
Pancreas allograft exocrine secretions can be drained in one of two manners, either into the small bowel (duodenoenterostomy) or urinary bladder (duodenocystostomy) (Figs. 24.2 and 24.3) [12].
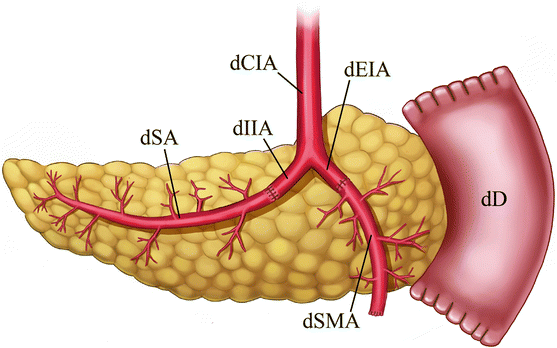
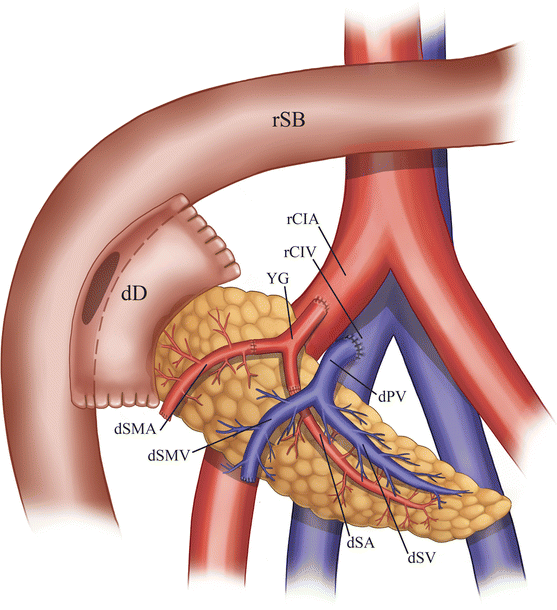
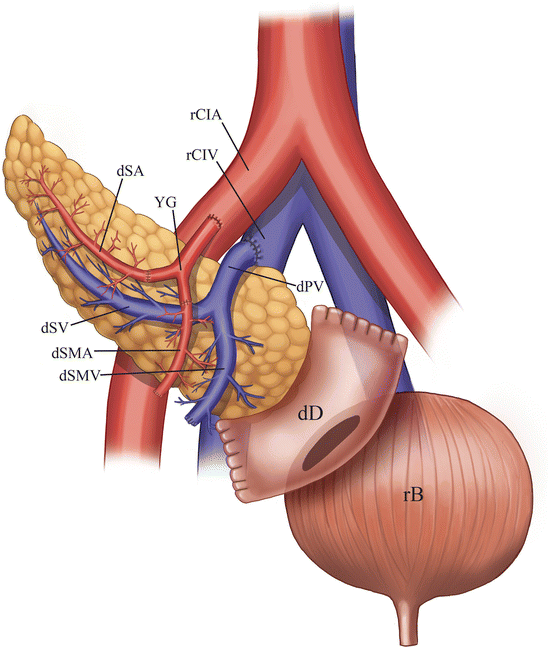

Fig. 24.1
Illustration of pancreas allograft Y-graft and arterial anastomoses. The internal iliac artery portion of the Y-graft is sutured to the splenic artery, while the external iliac artery portion is sutured to the superior mesenteric artery. dCIA donor common iliac artery, dEIA donor external iliac artery, dIIA donor internal iliac artery, dSA donor splenic artery, dSMA donor superior mesenteric artery, dD donor duodenum

Fig. 24.2
Illustration of pancreas allograft with enteric drainage. The donor duodenum is anastomosed to a loop of recipient small intestine. The donor common iliac artery portion of the Y-graft is anastomosed to the recipient common iliac artery, while the donor main portal vein is anastomosed to the recipient common iliac vein. dSA donor splenic artery, dSV recipient splenic vein, dSMA donor superior mesenteric artery, dSMV recipient superior mesenteric vein, dPV donor PV, dD donor duodenum, rSB recipient small bowel, rCIA recipient common iliac artery, rCIV recipient common iliac vein, yG Y-graft

Fig. 24.3
Illustration of a pancreas allograft with urinary bladder drainage. The donor duodenum is anastomosed to the urinary bladder. The donor common iliac artery portion of the Y-graft is anastomosed to the recipient common iliac artery, while the donor main portal vein is anastomosed to the recipient common iliac vein. dSA donor splenic artery, dSV recipient splenic vein, dSMA donor superior mesenteric artery, dSMV recipient superior mesenteric vein, dPV donor PV, dD donor duodenum, rCIA recipient common iliac artery, rCIV recipient common iliac vein, yG Y-graft, rB recipient bladder
Enteric drainage is accomplished by anastomosing the donor duodenum to a loop of recipient small intestine (usually 30–40 cm distal to the ligament of Treitz) in a side-to-side fashion [12, 13]. Pancreas exocrine secretions are less frequently drained into the urinary tract by anastomosing the donor duodenum to the urinary bladder dome [12].
While urinary bladder drainage was previously favored, most pancreas transplantation procedures are now performed employing enteric drainage [1]. Advantages of enteric drainage include physiologic management of pancreatic exocrine secretions, fewer metabolic complications (due to less bicarbonate loss), no associated pancreatitis due to reflux of urine into the allograft, and no urinary tract complications [12, 14–17]. The primary disadvantage of enteric drainage is that urine amylase concentration cannot be followed over time to assess graft function and rejection [12, 15]. Because of a variety of complications, a substantial number of pancreas allografts managed with urinary bladder drainage require eventual conversion to enteric drainage.
Imaging of pancreas transplants is usually performed in the setting of abnormal allograft function or suspected allograft-related complication. Ultrasound, CT, and MRI can be used to assess the transplant pancreas. As always, imaging findings should be interpreted with knowledge of both the patient’s clinical condition and available pertinent laboratory results (e.g., serum amylase/lipase, WBC count).
Ultrasound is the most commonly used imaging modality for pancreas transplant assessment [15]. Gray-scale ultrasound demonstrates the allograft as well as adjacent structures. Doppler imaging can be used to confirm the presence of blood flow within the allograft as well as confirm patency of allograft major arterial and venous structures [15]. Vascular thrombosis, stenosis, pseudoaneurysm, and arteriovenous fistula can all be detected with Doppler ultrasound. In the future, contrast-enhanced ultrasound may play an increasing role in assessing certain allograft-related vascular complications, such as arterial thrombosis [18]. Contrast-enhanced ultrasound has also been used preoperatively to evaluate the vascular integrity of harvested pancreas allografts ex vivo prior to placement in the recipient [19].
Ultrasound has several advantages over other imaging modalities, including its nonionizing nature, portability, lack of need for oral and intravenous contrast materials, and the fact it provides excellent spatial resolution when assessing superficial structures using a linear high-frequency transducer. Disadvantages of ultrasound include its operator dependence and the fact that the allograft may be poorly visualized due to overlying bowel gas or bandages [15]. In the early postoperative setting, up to 20 % of allografts may not be visible for a variety of reasons, such as overlying bandages and adjacent hematoma [15, 16].
Computed Tomography
Current generation multidetector CT scanners also excellently depict pancreas allografts and associated complications [13, 20]. Pancreas allografts are best assessed by reviewing very thin-section axial images as well as isotropic coronal and sagittal reformatted images. Both oral and intravenous contrast materials assist with distinguishing the transplanted pancreas from adjacent bowel loops [14]. CT imaging in the arterial and venous phases may be helpful in identifying a variety of vascular complications [15]. The large field of view afforded by CT compared to ultrasound also helps determine the true extent of peripancreatic fluid collections as well as identify more distant intra-abdominal fluid collections [14]. CT imaging following the instillation of contrast material into the urinary bladder (CT cystography) may be helpful in the setting of suspected anastomotic or duodenal leak following duodenocystostomy. Disadvantages of CT include its ionizing nature, lack of portability, and the fact that intravenous contrast material may be contraindicated in certain patients due to kidney transplant dysfunction.
Magnetic Resonance Imaging
Although less commonly utilized, MRI can be used to evaluate pancreas allograft parenchyma as well as assess for a variety of related complications [21]. Standard precontrast and postcontrast MRI pulse sequences can be used to evaluate allograft parenchyma and intra-abdominal fluid collections. High-resolution three-dimensional (3D) MR angiography (MRA) can be used to accurately depict major allograft arterial and venous complications, including complete or partial arterial graft occlusion, stenosis of the arterial Y-graft, complete venous thrombosis, arteriovenous fistula, and pseudoaneurysm formation [21, 22]. MR pancreatography with secretin stimulation can be used to evaluate for rejection-related changes in allograft exocrine function as well as ductal changes in the setting of pancreatitis [21, 23]. Advantages of MRI include its nonionizing nature, excellent contrast resolution, multiplanar capabilities, and ability to assess larger vascular structures without intravenous contrast. Disadvantages of MRI include its higher cost, longer length of study, lack of portability, and the fact intravenous contrast material is contraindicated in patients with acute renal failure or chronic kidney disease with an estimated glomerular filtration rate less than 30 ml/min/1.73 m2 due to risk of nephrogenic systemic fibrosis material [14, 15, 22].
Imaging of the Normal Pancreas Allograft
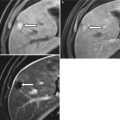
At ultrasound, the normal pancreas allograft demonstrates homogeneous echotexture and appears hypoechoic when compared to the normal pancreas as well as adjacent intra-abdominal omental and mesenteric fat (Fig. 24.4a) [14, 16]. The margins of the transplanted pancreas may appear somewhat ill defined, as the pancreas lacks a true capsule [14–16]. Occasionally, normal pancreas allografts may be difficult to be distinguished from adjacent bowel [14]. Doppler ultrasound demonstrates the presence of blood flow within the allograft and its major arterial and venous structures. In particular, power Doppler excellently depicts the donor Y-graft and other major vessels (Fig. 24.4b) [15].
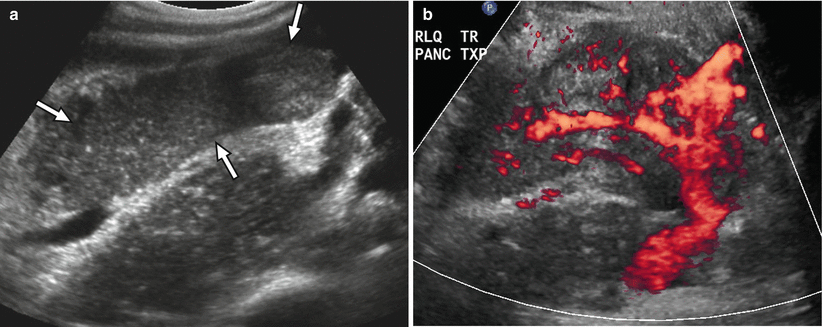

Fig. 24.4
Ultrasound appearance of normal transplant pancreas. (a) Gray-scale ultrasound image shows a homogeneous right lower quadrant pancreas allograft (arrows) that appears hypoechoic to adjacent intra-abdominal fat. (b) Power Doppler image confirms the presence of blood flow within the allograft, including within the Y-graft
In cases where the allograft is difficult to identify from adjacent structures, Doppler ultrasound may assist in allograft recognition [14]. The presence of peripancreatic fluid may also improve allograft conspicuity [15].
At CT, the normal pancreas allograft appears relatively well defined, demonstrating soft tissue attenuation on noncontrast imaging and homogeneously enhancing on postcontrast imaging (Fig. 24.5) [14]. Postcontrast images show intravascular contrast material opacifying the major arterial and venous structures flowing into and draining the allograft. The main pancreatic duct may not be visualized or may appear slightly prominent [16].
The normal pancreas allograft appears homogeneous and relatively well defined on both precontrast and postcontrast MRI pulse sequences (Fig. 24.6). Transplant pancreas parenchyma is usually isointense to normal renal cortex on unenhanced T1-weighted imaging and of intermediate signal on T2-weighted imaging [14]. Postcontrast images should reveal intravascular contrast material filling the major arterial and venous structures flowing into and draining the allograft [22].
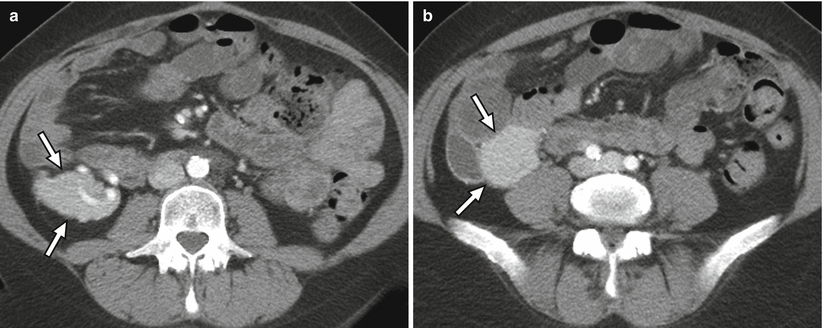
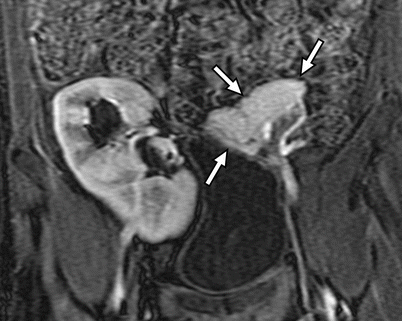

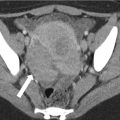
Fig. 24.5
CT appearance of normal transplant pancreas. (a, b) Axial contrast-enhanced CT images demonstrate a homogeneously enhancing pancreas allograft (arrows) in the right lower quadrant. Intravascular contrast material can be seen in patent donor splenic and superior mesenteric arteries

Fig. 24.6
MRI appearance of normal transplant pancreas. Coronal contrast-enhanced 3D spoiled gradient recalled echo image with fat saturation reveals a homogeneously enhancing pancreas allograft (arrows) in the left lower quadrant. Major transplant vessels can be visualized and are patent. An enhancing renal allograft is present in the right lower quadrant
Pancreas Transplantation Complications (Table 24.1)
Table 24.1
Summary of pancreas transplant complication
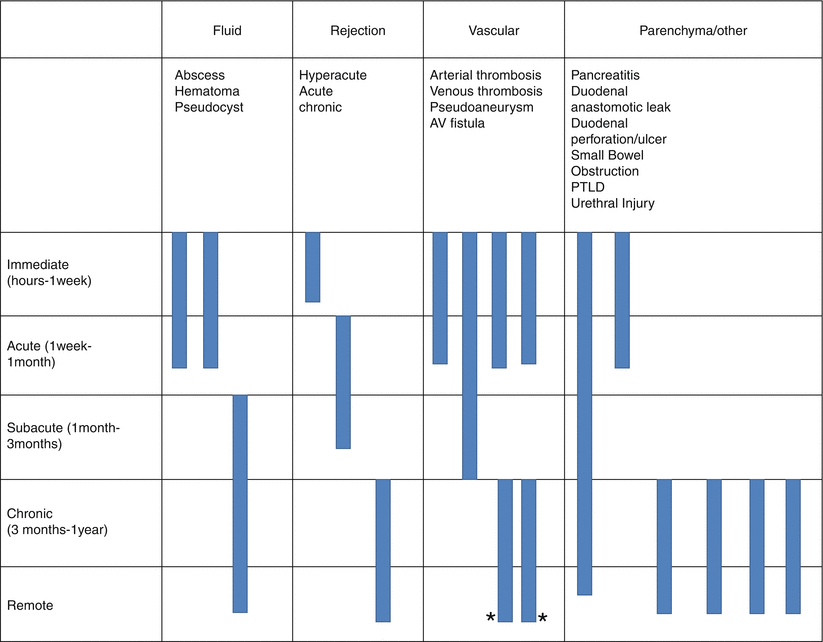
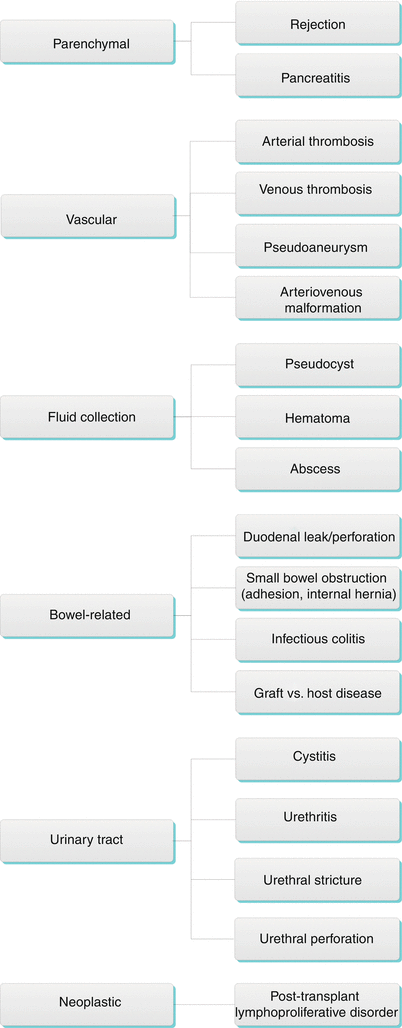
Algorithm 24.3 Complications of Pancreas Transplantation
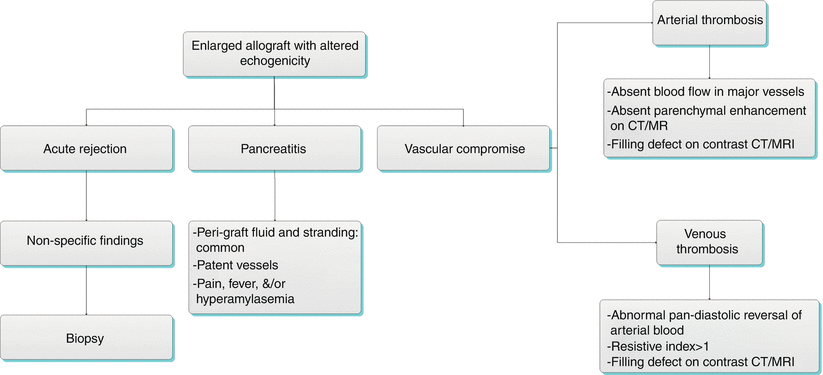
Algorithm 24.4 Diagnostic approach of enlarged allograft with altered echogenicity
Parenchymal
Allograft Rejection
Immune system-mediated rejection is a common cause of pancreas allograft failure that may be hyperacute, acute, or chronic. The rate of acute rejection is approximately 15 %, while the rate of chronic rejection in allografts surviving greater than 6 months is approximately 25 % [11]. Imaging generally plays a limited role in the appraisal pancreas allograft rejection due to a general lack of sensitivity and specificity.
Gray-scale ultrasound in the acute rejection setting may mimic acute pancreatitis or vascular compromise with the allograft appearing enlarged and having altered echogenicity and echotexture [15, 24]. In the setting of chronic rejection, the allograft may appear atrophic (Fig. 24.7a). There is little role for spectral Doppler in the evaluation of pancreas allograft acute rejection. In a study by Wong et al. [24], a spectral Doppler resistive index greater than 0.7 had sensitivity for acute rejection of only 20 %. Nelson et al. [25] compared the results of 40 transplant pancreas biopsies with baseline resistive index measurements and identified no statistically difference in mean resistive index for individuals with no, mild, or moderate rejection. In fact, neither the absolute resistive index value nor any relative increase in resistive index correlated with acute rejection at biopsy, although an absolute increase in resistive index was commonly observed in chronic rejection (Fig. 24.7b) [25]. It has been suggested that the lack of correlation between elevated resistive index and acute rejection may be because the transplant pancreas lacks a true capsule [15].
At CT and MRI, rejection may present as abnormal pancreas allograft enlargement or atrophy [14]. The allograft may also appear heterogeneous and abnormally hypoenhanced [14, 21, 26]. The allograft may appear either diffusely or focally abnormally hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging in the setting of acute rejection [14, 21]. With chronic rejection, the allograft may appear hypointense on both T1-weighted and T2-weighted imaging due to the presence of fibrosis [21]. MR pancreatography with secretin augmentation, a form of functional MRI, can be used to demonstrate decreased pancreatic juice secretion due to allograft exocrine dysfunction, a finding that can be observed in the setting of rejection [21, 23]. Abnormal main pancreatic duct compliance, diagnosed when the main pancreatic duct fails to increase in diameter with secretin, may also suggest allograft dysfunction [21].
Because imaging and laboratory biochemical testing are both commonly insensitive and nonspecific for the diagnosis of pancreas allograft rejection, the presence of rejection is best confirmed with percutaneous biopsy and histopathologic assessment [14, 25, 27]. Wong et al. [24] concluded that image-guided percutaneous biopsy is superior to a combination of gray-scale and Doppler ultrasound for the detection of rejection and that a normal resistive index should not preclude biopsy. A study by Atwell et al. [27] documented that ultrasound-guided percutaneous pancreas allograft biopsy obtains adequate diagnostic tissue in greater than 90 % of procedures and that this method of tissue sampling has a very low complication rate.
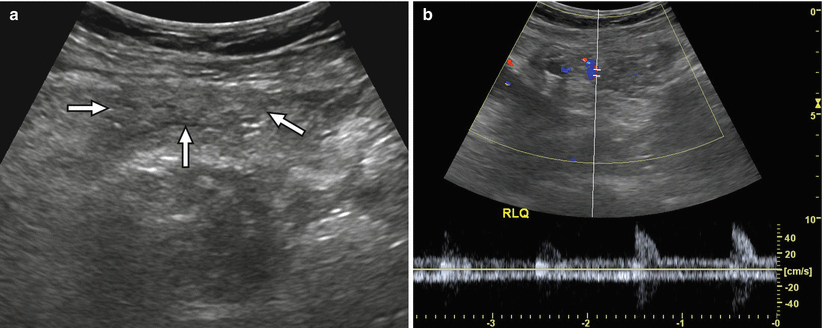

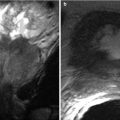
Fig. 24.7
A 54-year-old man worsening hyperglycemia due to transplant pancreas chronic rejection. (a) Gray-scale ultrasound image shows a shrunken, heterogeneous pancreas allograft (arrows) in the right lower quadrant. (b) Spectral Doppler waveforms acquired within the allograft demonstrate an abnormally elevated resistive index. Chronic rejection was confirmed at biopsy
Allograft Pancreatitis
Acute pancreatitis is a common early complication of pancreas transplantation, affecting approximately 10 % of allografts [16]. Pancreatitis in the early postoperative setting is likely due to multiple factors, including warm ischemia, handling of the allograft, and reperfusion injury [14, 20]. Late pancreatitis occurring more than 3 months following the transplantation procedure can also occur (for example, due to an anastomotic stricture or reflux of urine into the allograft in the setting of duodenocystostomy), and episodes may be multiple in up to 14 % of individuals [9, 14]. Clinically, pancreatitis may present with pain in the region of the allograft, fever, and/or hyperamylasemia [9]. Supportive medical management is generally adequate, as pancreatitis has little demonstrable effect on long-term allograft function and survival [9].
Imaging can be used to both help confirm the diagnosis of allograft pancreatitis and exclude other allograft-related complications. Allografts may appear normal at ultrasound in the setting of early or mild pancreatitis. In more severe instances, the allograft may appear either focally or diffusely enlarged with abnormally heterogeneous echogenicity and echotexture due to the presence of edema and inflammation (Fig. 24.8a). Peri-allograft fluid is commonly present, and Doppler signal should be present within the allograft and its major vascular structures (Fig. 24.8b). The absence of Doppler signal within the allograft in the setting of very severe pancreatitis should be considered suspicious for necrosis [16].
At CT and MRI, pancreatitis commonly presents with abnormal allograft enlargement and heterogeneous postcontrast enhancement (Fig. 24.8c) [14]. Inflammatory fat stranding and fluid may be observed adjacent to the allograft [9]. At MRI, the allograft may demonstrate abnormal T1-weighted signal hypointensity and T2-weighted signal hyperintensity. Gas within the allograft and absent postcontrast enhancement at CT and MRI in the setting of severe acute pancreatitis are concerning for allograft necrosis [16].
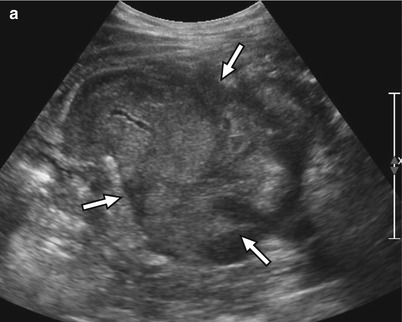
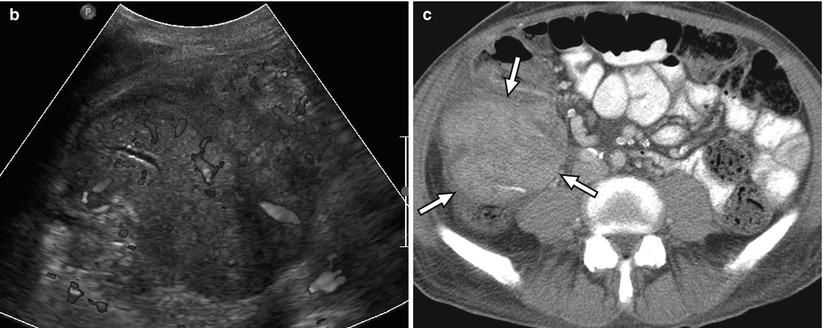


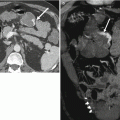
Fig. 24.8




A 59-year-old man with right lower quadrant tenderness, fever, and elevated serum amylase/lipase due to transplant pancreatitis. (a) Gray-scale ultrasound image shows a heterogeneous, markedly enlarged pancreas allograft (arrows) in the right lower quadrant. A small amount of peripancreatic fluid is also present. (b) Color Doppler image confirms the presence of blood flow within the allograft and adjacent mild hyperemia. (c) Axial contrast-enhanced CT image performed the following day demonstrates an enlarged, heterogeneously enhancing right lower quadrant pancreas allograft (arrows) with adjacent inflammatory changes, consistent with acute pancreatitis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree