Weakening of the female pelvic floor is a prevalent and debilitating disorder. It results in abnormal descent of the urinary bladder, the uterovaginal vault, and the rectum, resulting in urinary continence, fecal incontinence, and pelvic organ prolapse. Pelvic floor weakening affects approximately 50% of women older than 50 years at a direct annual cost of $12 billion. It is a major health issue in older women, as shown by the 11.1% lifetime risk for undergoing a single operation for pelvic organ prolapse and urinary incontinence, as well as the large proportion of reoperations.
Pelvic floor weakness has many complex causes. The risk factors for pelvic floor dysfunction include pregnancy, multiparity, advanced age, menopause, obesity, connective tissue disorders, smoking, chronic obstructive pulmonary disease, and any other factors that result in a chronic increase in intraabdominal pressure. A consensus conference statement from the National Institutes of Health concluded that age, sex, and vaginal parity are established risk factors. Although epidemiologic evidence supports the relation between vaginal delivery and pelvic floor dysfunction, not all women who undergo vaginal delivery develop pelvic floor dysfunction, and not all nulliparous women are free from pelvic floor dysfunction. Data and electromyographic studies also suggest that vaginal delivery causes neuromuscular damage to the pelvic floor well before the onset of pelvic floor dysfunction.
The support structures of the female pelvis consist of a complex network of pelvic muscles, fascia, and ligaments. Weakness of these structures results in abnormal descent of the pelvic floor organs and debilitating symptoms related to urinary or bowel incontinence, sexual dysfunction, and pelvic organ prolapse.
Normal Anatomy
General Anatomic Descriptions
The pelvic floor is divided into three compartments ( Figure 27-1 ). The anterior compartment contains the urinary bladder and the urethra; the middle compartment contains the uterus, cervix, and vagina; and the posterior compartment contains the rectum. The support for these structures arises from the attachment of the muscles, fascia, and ligaments to the bony pelvis. Magnetic resonance imaging (MRI) allows visualization of all three compartments and is extremely useful in assessing women who have symptoms of multicompartment prolapse before complex pelvic floor surgery is undertaken. The vagina, being the middle viscera and from its lateral attachments to the pelvic sidewalls via the ligaments, is the divider that determines the nature of any pelvic organ prolapse.
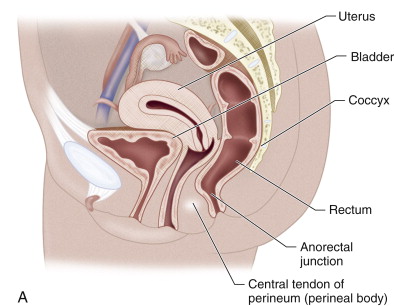



The pelvic fascia, pelvic floor musculature, and fascial condensations called ligaments are the primary supporting structures of the female pelvis. The endopelvic fascia is the most superior layer and forms a continuous sheet extending cephalad from the uterine artery to the point at which the vagina fuses with the levator muscles below. The endopelvic fascia covers the levator ani muscles and the pelvic viscera in a continuous sheet. Laterally the condensation of the endopelvic fascia forms the arcus tendineus, providing lateral support to and anchoring the levator ani muscles. The endopelvic fascia also attaches the cervix and vagina to the pelvic sidewall via the elastic condensations known as the parametrium and paracolpium, respectively. The parametrium is made up of the cardinal and uterosacral ligaments and provides support to the body of the uterus. The paracolpium stretches the vagina transversely between the urinary bladder and the rectum. The endopelvic fascia forms a supportive layer—the pubocervical fascia—between the pubis, the urinary bladder, and the anterior vaginal wall. Similarly, posteriorly the endopelvic fascia forms a supportive layer—the rectovaginal fascia—between the posterior vaginal wall and the rectum that prevents the rectum from protruding forward and the bowel from herniating inferiorly. These fascial condensations are not well visualized on conventional MRI; their defects may be inferred indirectly through secondary findings. They may be visualized with an endovaginal coil that is placed near the target organ and allows higher resolution and signal-to-noise ratio (SNR) than a surface or body coil and can therefore provide more detailed visualization of fine structures. This is especially useful for evaluation of the urethra and its surrounding structures: anteriorly to the urethra, posteriorly to the perineal body, and laterally with the levator ani muscles.
The levator ani muscles lie deep in relation to the endopelvic fascia. The two components of the levator ani that provide the major support to the pelvic organs are the puborectalis and the iliococcygeus muscles. The puborectalis forms a sling around the rectum and plays an important role in apposing the orifices of the pelvic floor, as well as elevating the bladder neck and compressing it against the pubic symphysis. The iliococcygeus has a horizontal orientation, arises from the external anal sphincter, and fans out laterally, attaching to the arcus tendineus. Posteriorly and in the midline, the iliococcygeus condenses to form a firm raphe anterior to the coccyx known as the levator plate . The iliococcygeus muscle acts as an important physical barrier, preventing posterior compartment prolapse. The muscles of the pelvic floor and levator plate are well visualized on MRI.
The perineal membrane lies inferior to the levator ani muscles and separates the vagina and rectum. It is a dense structure and is the point of insertion of five muscles: the deep transverse perineal muscle, the superficial muscles of the perineal membrane, the external urethral sphincter, the external anal sphincter, and the levator ani. The perineal body prevents expansion of the urogenital hiatus, which is the opening in the levator ani muscle groups through which the urethra, vagina, and rectum course; it is also the orifice through which pelvic organ prolapse occurs. The perineal membrane may be damaged during vaginal delivery via an episiotomy.
Etiology and Pathophysiology
It is the weakness of these supporting muscles, fascia, and ligaments that results in pelvic floor relaxation. This weakness progresses with age and may be related to menopause and hypoestrogenemic states. Loss of support to the urinary bladder and the urethra results in prolapse of the urinary bladder and protrusion of the anterior vaginal wall, forming a cystocele, which may result in urinary incontinence. Weakness of the parametrium and paracolpium causes prolapse of the cervix and uterus, and weakness of the rectovaginal fascia results in prolapse of the rectum and protrusion of the posterior vaginal wall, forming a rectocele, and may result in fecal incontinence. Prolapse of the small bowel through the rectovaginal fascia results in an enterocele. In patients who have undergone a hysterectomy, prolapse of the vaginal apex can arise because of weakness of the paracolpium, resulting in apical prolapse.
Manifestations of Disease
Simple stress incontinence is defined as unwanted urination with increased abdominal pressure such as a cough or laughter. It is caused by a failure of apposition of the urethral walls coupled with descent of the anterior compartment of the pelvis. The bladder neck often extends inferior to the symphysis, forming a cystocele. Rotation of the urethra anteriorly usually indicates significant ligamentous tear. When no cystocele is present, imaging is often not performed.
Detrusor instability, also called urge incontinence , is unwanted bladder contractions leading to urinary leakage. It occurs most often in elderly patients, often in association with stress incontinence. Patients may associate the incontinence episodes with specific actions such as opening a door or holding car keys. When necessary, diagnosis is made with videourodynamics. In this procedure, pressure-sensitive bladder and rectal catheters are placed. The bladder catheter (b) measures the internal pressure, and the rectal catheter (c) measures the abdominal pressure. The detrusor pressure is defined as (c) − (b). The detrusor muscle should demonstrate a smooth and slow increase in pressure as the bladder fills. When a contraction occurs, a spike is seen in the pressure tracing.
Prolapse of the bladder (cystocele) and vaginal vault is a common scenario in the perimenopausal or posthysterectomy patient. These are often identified as a perineal bulge on physical examination, although small cystoceles may only become evident with the patient in the standing position. The apex of the vaginal cuff or uterus is mobile, and defects may be identified in the fascia of the pelvic floor. Depending on the confidence of the examining physician, imaging may be performed to plan corrective surgery.
Weakening or loss of the rectovaginal fascia and other posterior fascial planes leads to abnormal descent of the sigmoid, rectum, or small bowel—sigmoidocele, rectocele, and enterocele, respectively. Symptoms include incomplete emptying of the colon, constipation, and fecal incontinence. To identify which portions of the bowel are involved, evaluation of the anal sphincter and pelvic floor is usually performed.
Symptomatic global pelvic floor prolapse is failure of all the fascial condensations and often the musculature of the pelvic floor. The pelvic organs are usually below the pubic symphysis at rest. This constellation of physical findings is associated with constipation and repetitive straining. Because these cases will often require a team approach consisting of a urologist, gynecologist, and colorectal surgeon to reapproximate supporting ligaments, elevate the bowel and bladder, and repair muscular defects, preoperative imaging is valuable.
Imaging Indications and Algorithm
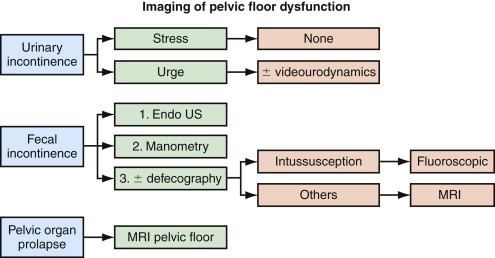
An imaging algorithm for women with pelvic floor dysfunction is presented in Figure 27-2 . In general, simple urinary incontinence requires no imaging and will not be discussed further. Women with cystocele, vaginal vault or uterine prolapse, fecal incontinence, chronic constipation, and/or rectal prolapse are good candidates for imaging. Women with pathology involving multiple pelvic floor compartments and those with recurrent symptoms after surgery should undergo imaging to identify specific anatomic defects before repeat reconstructive repair.
Radiography
Fluoroscopic cystography is rarely performed in the radiology department. Exceptions include trauma to the bladder and evaluation of suspected urinary reflux.
Videourodynamics may be used to diagnose urge incontinence. A bladder contraction seen on the screen is a visual correlate to increased detrusor pressure.
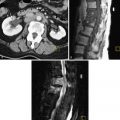
Fluoroscopic defecography is a good technique for evaluation of isolated rectal prolapsed or intussusception, particularly in the elderly population. The patient first drinks two 8-oz cups of barium solution. After a 1-hour hiatus to coat the small bowel, the vagina is opacified with a tampon soaked in iodine-based water-soluble contrast agent. Finally, with the patient in the left lateral decubitus position, the rectum is filled with barium paste. The patient is then placed on a radiolucent commode chair parallel to the fluoroscopic table and perpendicular to the radiation beam. Spot films are obtained at rest, with pelvic floor contraction, with cough, strain, and finally during defecation. The entire procedure requires approximately 10 minutes to perform. An advantage of the technique is imaging in the upright, physiologic position, which eases defecation. Pelvic floor hernias, rectal intussusceptions, and sphincter dyssynergia are also well depicted. Disadvantages include the invasive nature of the preparation, requirement for a commode chair, and a relatively high dose of radiation. For this reason, it is no longer performed in most hospitals.
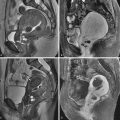
Review of the initial images shows location of the rectum and small bowel at rest. The orientation of the vagina should remain vertical throughout the study ( Figure 27-3 ). Contraction images show shortening and thickening of the puborectalis and a decrease in the anorectal angle. Absolute values of the angle are not recorded because there is significant overlap in symptomatic and asymptomatic women.

Straining images may demonstrate fecal incontinence or rotation of the vagina, indicating loss of fascial support. Images obtained during defecation may show prolapse of the rectum or sigmoid below the rectal hiatus, enterocele with bowel loops extending into the pouch of Douglas, or rectocele ( Figure 27-4 ). Rectoceles are defined as outpouchings of rectal tissue that extend 2 cm or more beyond the expected wall of the rectum that fill with fecal material. A persistent collection of contrast material after defecation gives the patient a feeling of incomplete evacuation. Many women manually empty rectoceles.

Ultrasound
Transperineal ultrasound is an excellent tool for identifying funneling of the bladder neck, which occurs in stress incontinence and urethral diverticula. It is not commonly used in North America but is often used in assessment of stress incontinence in Europe.
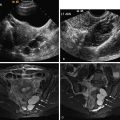
Transrectal ultrasound is the test of choice for evaluation of the anal sphincter. This is part of the diagnostic assessment of patients with fecal incontinence, usually preceding manometry to assess rectal tone. The normal anal sphincter is 2.5 to 3.0 cm in length. An oval probe encased in gel is placed in the sphincter, and circumferential images are obtained along its length. The most superior portion of the sphincter is identified by the iliococcygeus muscles. Just inferior to this, two concentric rings are identified. The inner ring is hypoechoic and is the internal anal sphincter; the outer ring originates at the midlevel of the anal canal, is hyperechoic to muscle, and is the external anal sphincter ( Figure 27-5 ). Defects in these rings most commonly occur anteriorly in women with fecal incontinence, likely secondary to episiotomy or rectal tear during childbirth ( Figure 27-6 ).