Rajendra N. Solanki, Chetan Mehta, Nirja Desai, Digish Vaghela The anatomical neck is divided into multiple mucosal and extra mucosal spaces. The infrahyoid neck has tightly bound fascial compartments, whereas the suprahyoid neck has comparatively imperceptible fascial boundaries resulting in numerous bays in the retrovisceral, peripharyngeal regions and the floor of the mouth. We discuss the significant space contributing to the floor of the mouth, i.e. the submandibular space. Pathologies growing in these spaces are a common clinical dilemma for the clinicians, presenting as a neck swelling or mucosal ulcer and imaging plays an important role in the diagnosis, disease extent and patient management. The components of submandibular space are derivatives of the first, and minimally, of the second branchial arch. Reasonably, syndromes with first branchial arch anomalies like Treacher Collins and Pierre Robins syndrome have a high incidence of submandibular gland aplasia/hypoplasia. Submandibular and sublingual gland anlages start developing during the eighth week of gestation, much later than parotid glands (sixth week). However, parotid glands get enclosed in cervical fascial layers later than the other major salivary glands. This fact is essential as lymphoid tissue gets entrapped in the unencapsulated parotid parenchyma resulting in the less frequent involvement of the submandibular gland in pathologies like lymphomas, benign lymphoepithelial lesions of HIV and reactive lymphoid hyperplasia as compared to the parotid gland. Submandibular space (SMS) is a small region bounded medially by the anterior belly of the digastric muscle, anterolaterally by the mandible, superiorly mylohyoid sling, posteriorly by the hyoid bone and inferolaterally by skin, superficial fascia, platysma and the superficial layer of deep cervical fascia. Mylohyoid sling is the crucial imaging landmark for surgical planning as pathologies below the sling mostly require a neck incision, while those above the sling require an intraoral approach. SMS is a closed space barring two openings, first located posteriorly at the free edge of the mylohyoid, where the submandibular and sublingual spaces communicate freely, also having potential communication with the inferior aspect of the parapharyngeal space. The other being mylohyoid boutonniere, a frequently found focal defect in the mylohyoid muscle, containing fat and allowing passage of vessels. When the boutonniere contains heterotopic salivary tissue, it may appear as a pseudolesion on imaging (Table 3.16.1). Contained within the submandibular space is the superficial portion of the submandibular salivary gland, level Ib lymph nodes, including the node of Stahr (which lies on the facial artery) and fat. The principal component of the submandibular space is the submandibular gland, wrapping around the mylohyoid muscle like the letter C, with the larger superficial lobe inferolateral to the mylohyoid, running parallel to the anterior digastric belly, and the smaller deep lobe, along with the hilum, lying superomedial to the mylohyoid. The facial artery and vein and inferior loop of the hypoglossal nerve, also course through this space. The anterior facial vein is an important anatomical landmark for differentiating a salivary gland mass lesion from a lymph node or other masses lateral to the submandibular gland. The gland has some crucial vascular landmarks nearby. The facial artery and vein pass lateral to the gland in the submandibular space, with the lingular artery and vein crossing over the mylohyoid, medial to the deep lobe of the gland through the sublingual space. The anterior branch of the retromandibular vein courses between the anteroinferior parotid and the dorsal submandibular gland. The submandibular duct (Wharton duct) is an approximately 5 cm long thin-walled duct that emerges from the hilum at the level of the posterior free border of the mylohyoid muscle, takes a turn a turn around it and extends anteriorly and medially with an upward inclination, passing medial to the sublingual gland and lateral to the genioglossus muscle, and terminates at the sublingual papilla along the medial part of the sublingual gland. Pathologies in SMS can be divided into four broad categories: However, four categories of submandibular space pathologies present with non-specific signs and symptoms (most common being swelling in the submandibular triangle of the neck). For example, the clinical picture of submandibular sialadenitis may be secondary to the obstruction of Wharton’s duct by a tumour growing in the anterior floor of the mouth. Imaging features play an important role in identifying the lesion, mapping its extent and characterization based on imaging, followed by confirmation with guided biopsy. This aids the physician with patient counseling and initiating prompt and appropriate treatment. Infective processes of the submandibular space are diagnosed clinically, the initial presentation being pain, tenderness and swelling, and in later stages with dysphagia and stridor. Imaging helps to identify the source of infection (tooth, salivary gland, adjacent spaces, overlying soft tissues, or the mandible) of the infection, accurately define the extent and confirm the presence/absence of a frank drainable abscess (Figs 3.16.1 and 3.16.2). Sialolithiasis most commonly involves the submandibular glands (86%–92%) due to the high mucus content of its secretion, long-tortuous course of the exiting duct and its narrow lumen. Common sites of calculus impaction are at the bend at the posterior mylohyoid border, at the terminal ostium, less commonly in the mid-portion of the duct, at the hilum, and in the gland parenchyma. Contrary to parotid sialoliths, which are commonly radiolucent (35%–40%), submandibular sialoliths are rarely so (15%–20%). Duct calculi (Fig. 3.16.3A–C) are the most common cause of submandibular sialadenitis. The other common causes of sialadenitis include duct strictures, a mass occluding the duct lumen, and rarely, mucus plugs in Kussmaul’s disease (sialodochitis fibrinosa). It is diffuse soft tissue cellulitis involving bilateral submandibular and sublingual spaces, spreading via the fascial planes rather than lymphatics, most commonly following dental infections (apical abscess and pericoronitis). Its potential complications include the spread of infection to Apart from the extent of involvement, CECT also helps in The lingual aspect of the molar region of the mandible is the thinnest cortex; hence molar odontogenic infection easily perforates this site to spread into the adjacent masticator, submandibular and sublingual spaces, both of which communicate with each other and with the parapharyngeal spaces. Also, both the sublingual spaces are contiguous, allowing infection to spread.
3.16: Imaging of submandibular space
Introduction
Embryology
Anatomy
Pathologies
Infection, inflammations and obstructive causes
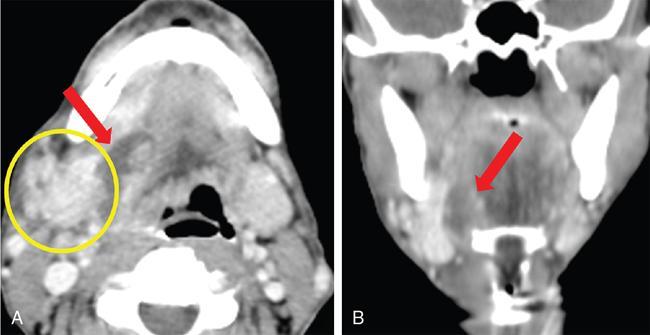
 ) in the right submandibular space. The right submandibular gland (
) in the right submandibular space. The right submandibular gland (  ) appears mildly enlarged and shows heterogeneous enhancement.
) appears mildly enlarged and shows heterogeneous enhancement.
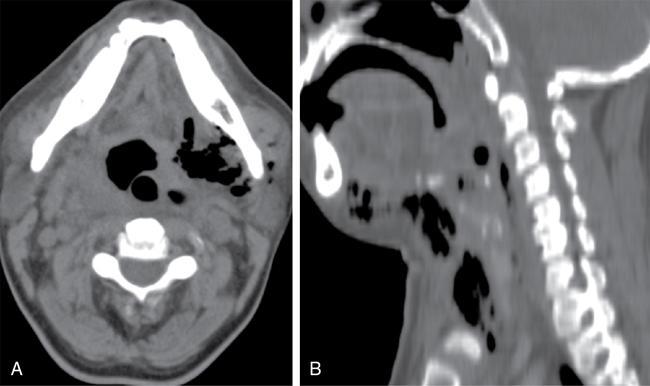
Sialadenitis
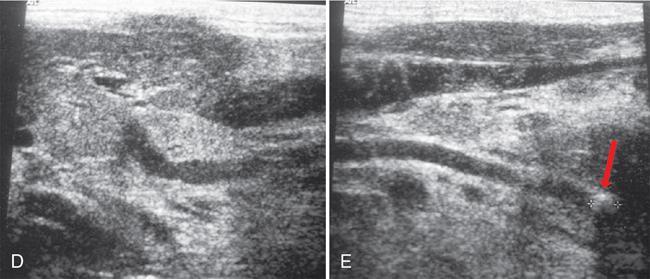
 ) in the submandibular duct with mild dilatation of the upstream duct (
) in the submandibular duct with mild dilatation of the upstream duct (  ) with an inflammed left submandibular gland (
) with an inflammed left submandibular gland (  ). (D & E): The hyperechoic area at distal SMG duct (
). (D & E): The hyperechoic area at distal SMG duct (  ) causing proximal duct dilatation; the affected SMG gland is hypoechoic and enlarged suggestive of sialoadenitis.
) causing proximal duct dilatation; the affected SMG gland is hypoechoic and enlarged suggestive of sialoadenitis.
Imaging features
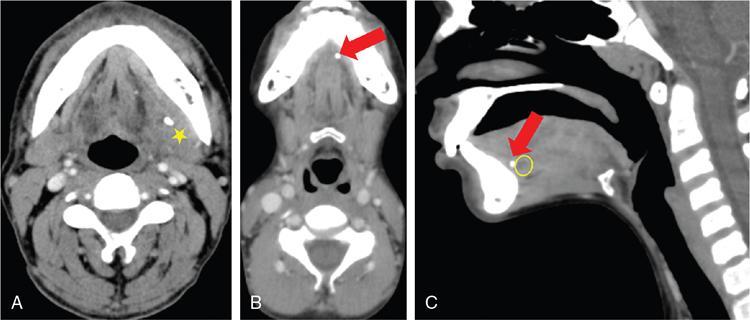
Ludwig’s angina (Fig. 3.16.4)
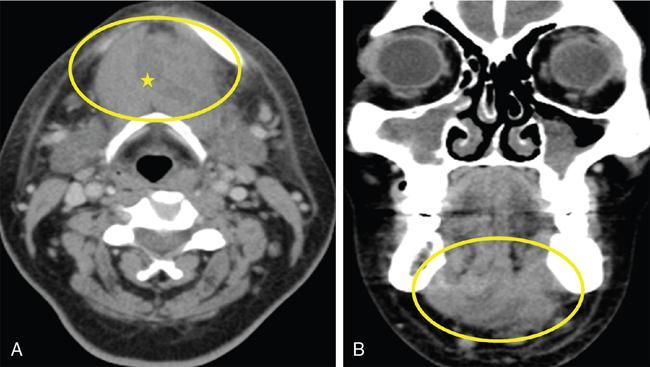
 ), with multiple coalesced hypodense non-enhancing collections (
), with multiple coalesced hypodense non-enhancing collections (  ) within, and some level Ia and Ib lymph nodes.
) within, and some level Ia and Ib lymph nodes.
Imaging features
Odontogenic infections
Imaging features
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine