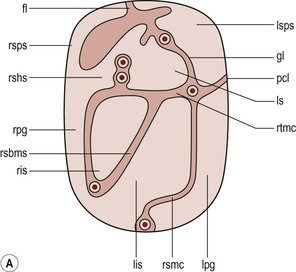
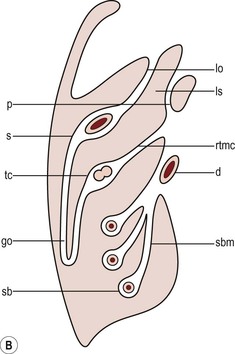
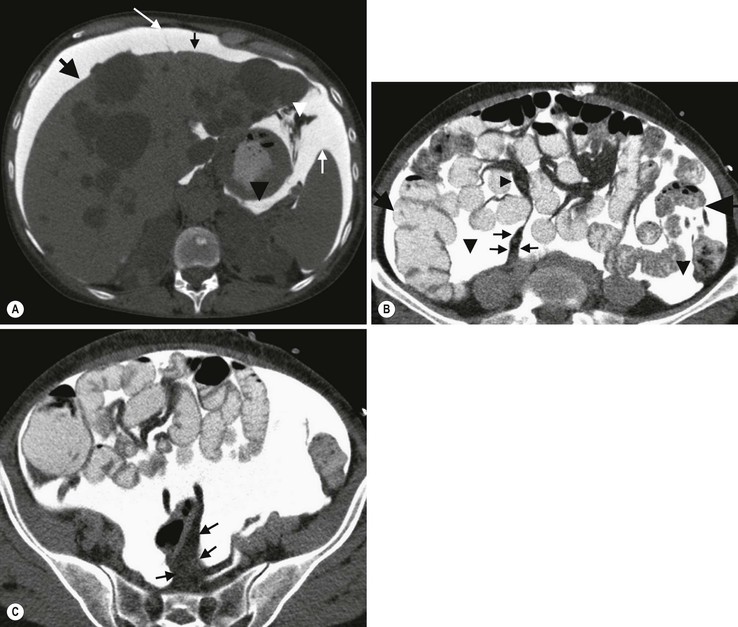
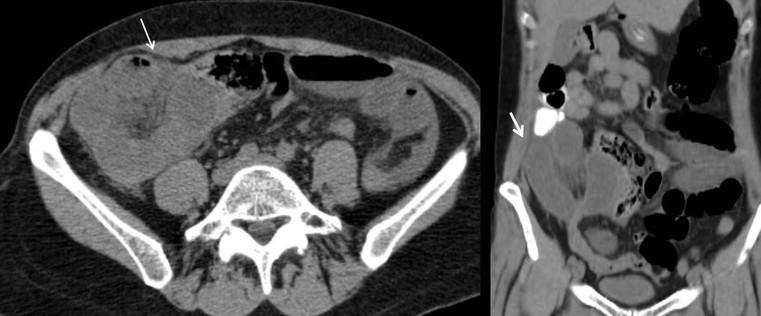
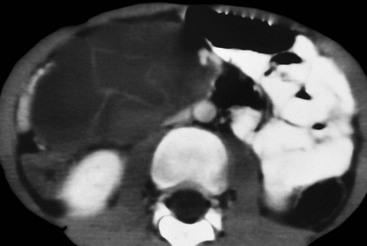
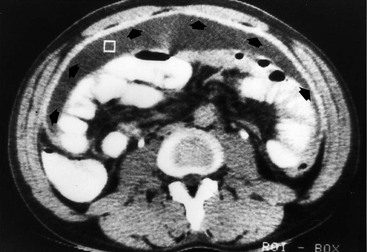
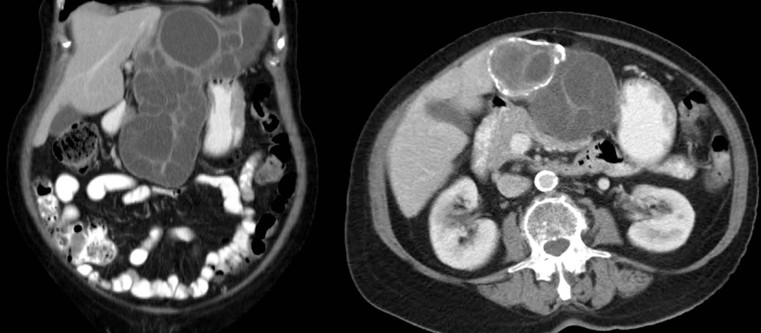
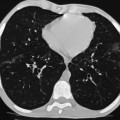
Nicholas Gourtsoyiannis, Panos Prassopoulos, Maria Daskalogiannaki The peritoneum is the largest serous membrane in the body. It consists of the parietal peritoneum, which lines the abdominal wall, and the visceral peritoneum, which envelops hollow and solid abdominal viscera. Between these two layers lies the peritoneal cavity. Peritoneal reflections interconnect the organs and viscera enclosed within the peritoneal cavity. The name of a particular ligament corresponds to the two major structures that it joins, e.g. hepatoduodenal, splenorenal ligament. Ligaments that attach the stomach to other structures are termed ‘omenta’. The mesenteries connect a portion of bowel with the posterior abdominal wall. The fatty tissue enclosed by peritoneal folds is in anatomic continuity with the retroperitoneal and properitoneal tissues.1 A potential space, termed the ‘subperitoneal space’ is enclosed within the peritoneal membrane of the mesenteries, bridges the peritoneal cavity with the retroperitoneum and represents a significant pathway for the spread of disease from the retroperitoneum to the peritoneal cavity and vice versa.2 The potential peritoneal spaces and the peritoneal reflections act as boundaries for pathological processes but may also become conduits for the spread of disease.1 Typically, the peritoneal folds are not directly visible on cross-sectional imaging, but they can be identified by either their typical position or organ relationships or by the anatomical landmarks provided by their major constituent vessels. When they become thickened by oedema, inflammation or neoplastic infiltration, they can be directly recognised on computed tomography (CT) or magnetic resonance imaging (MRI). The peritoneal cavity is subdivided by peritoneal reflections into multiple compartments and recesses (Fig. 30-1). On cross-sectional imaging, the peritoneal spaces are not visualised unless they are distended by fluid (Fig. 30-2). The peritoneal cavity is divided into the supramesocolic and the inframesocolic compartments by the transverse colon and its mesentery.3 The supramesocolic space extends from the diaphragm to the transverse mesocolon. It is divided into right and left peritoneal compartments, which are arbitrarily subdivided into intercommunicating spaces. The right supramesocolic space (Fig. 30-1A) includes the right perihepatic space and the lesser sac.1 The right perihepatic space includes the subphrenic and subhepatic spaces. The subphrenic space extends over the diaphragmatic surface of the right lobe of the liver and it is limited on the left by the falciform ligament and posteromedially by the right coronary ligament, which forms the right lateral margin of the bare area of the liver. The subhepatic space, also called the hepatorenal fossa or Morison’s pouch, consists of the posteromedial continuation of the subphrenic space, extending between the liver and the right kidney. Gallbladder infections or collections after gallbladder surgery tend to accumulate in this space.2 The lesser sac is subdivided into a small superior recess and a larger inferior recess, by peritoneal reflection over the left gastric artery. The superior recess surrounds the caudate lobe of the liver and communicates with the right subhepatic space via the slit-like foramen of Winslow that is located between the inferior vena cava and portal vein. The larger inferior recess lies between the stomach, the visceral surface of the spleen and the pancreas. On the left it is bounded by the gastrosplenic ligament anteriorly and the splenorenal ligament posteriorly. Abnormalities of the transverse colon, pancreas, posterior wall of the stomach, duodenum and caudate lobe of the liver may extend into the lesser sac. The left supramesocolic space is subdivided into four intercommunicating compartments.1 The left anterior perihepatic space is bounded on the right by the falciform ligament, posteriorly by the liver surface and anteriorly by the diaphragm (Fig. 30-2A). It is mainly affected by lesions arising from the left lobe of the liver and the stomach.2 The left posterior perihepatic space, also called the gastrohepatic recess, follows the posterior margin of the lateral segments of the left hepatic lobe. It is in close proximity to the lesser curve of the stomach, the anterior wall of the duodenal bulb and the anterior wall of the gallbladder. Abnormalities in any of these organs may extend into this space.3 The left anterior subphrenic space lies between the anterior wall of the stomach and the left hemidiaphragm, communicating inferiorly with the left anterior perihepatic space. Fluid collections in this space may result from perforation of the stomach or the splenic flexure of the colon.4 The left posterior subphrenic or perisplenic space is the posterior extension of the anterior subphrenic space (Fig. 30-2A). The inframesocolic space is bordered superiorly by the transverse mesocolon and inferiorly by the pelvic rim. It contains the infracolic space and the paracolic gutters (Fig. 30-2B). The obliquely directed small-bowel mesentery, extending from the left upper midabdomen to the right iliac fossa, divides the infracolic space into a smaller right and a larger left space. The right infracolic space terminates at the ileocaecal junction. The left infracolic space is anatomically open to the pelvis except where it is restricted by the sigmoid mesocolon (Fig. 30-2C). The ascending and descending colon form the lateral borders of the right and left inframesocolic space, respectively. The paracolic gutters are located alongside the lateral borders of the ascending and descending colon. The right paracolic gutter is continuous with the right perihepatic space and with the intraperitoneal pelvic space. Cephalad continuation of the left paracolic gutter is partially restricted by the phrenicocolic ligament. The pelvic peritoneal cavity consists of the lateral paravesical spaces and the pouch of Douglas—the rectovesical space in men, the rectouterine space in women. The mesenteries are double-layered peritoneal folds enclosing either the small bowel or portions of the colon and connecting them to the posterior abdominal wall. They contain a variable amount of adipose tissue, the superior or inferior mesenteric arteries and their branches, the associated veins, lymphatic vessels and nerves. The small-bowel mesentery is a broad fan-shaped fold that suspends the jejunum and ileum from the posterior abdominal wall and contains the intestinal branches of the superior mesenteric vessels, lymph nodes, nerves and abundant fat.4 Its root originates at the duodenojejunal junction and extends downward in an oblique direction to the ileocaecal junction. The root is 15 cm long, while the intestinal border is 6–8 m in length. As a result, the mesentery has a pleated appearance along its intestinal border. The mesenteric folds are not discernible, unless they are separated by intervening fluid (Fig. 30-2B) or peritoneal thickening. Vasa recta can be identified within the fatty mesenteric tissue radiating in relationship to the mesenteric borders of small-bowel loops.3 The transverse mesocolon suspends the transverse colon from the posterior abdominal wall and provides an important route for the spread of disease across the mid-abdomen. On cross-sectional imaging, the transverse mesocolon can be identified as the fat-containing area extending from the uncinate process, the inferior border of the body and tail of the pancreas to the ventrally positioned transverse colon, containing the middle colic vessels. The sigmoid mesocolon attaches the sigmoid colon to the posterior pelvic wall (Fig. 30-2C) and contains sigmoid and haemorrhoidal vessels. It has an inverted V-shape configuration with its apex lying anterior to the bifurcation of the left common iliac artery. The greater omentum is a four-layered fold that descends from the greater curvature of the stomach, before turning superiorly again to insert into the anterosuperior aspect of the transverse colon. It provides an important pathway of disease spread from the greater curvature of the stomach to the transverse colon and vice versa. It has also an important role in limiting the spread of infectious diseases and confining bowel injuries, whereas it is a common site of involvement in metastatic peritoneal disease. On cross-sectional imaging, the greater omentum is identified as a fatty area extending behind the anterior abdominal wall, sometimes descending deep into the pelvis. There are portions of the greater omentum that are referred to with special names. The gastrocolic ligament is the segment of the greater omentum that links the stomach with the transverse colon. The duodenocolic ligament connects the first portion of the duodenum and the transverse colon. The gastrosplenic ligament extends from the stomach to the splenic hilum (Fig. 30-2A). The lesser omentum or gastrohepatic ligament extends from the lesser curvature of the stomach deep to the fissure for the ligamentum venosum, between the caudate and left hepatic lobes (Fig. 30-1B). It contains the left gastric artery, the coronary vein and the left gastric nodal chain. Disease processes from the stomach extending along the gastrohepatic ligament may invade the liver, as the areolar tissue within the ligament is continuous with the hepatic capsule.1 The inferior edge of the gastrohepatic ligament, known as the hepatoduodenal ligament, bridges the upper duodenal flexure to the porta hepatis and contains the common hepatic duct, common bile duct, hepatic artery and portal vein. The normal peritoneal cavity contains only a small amount of serous fluid—less than 100 mL. Free-fluid accumulation exceeding this amount is considered to be ascites. Accumulation of fluid in the peritoneal cavity is not a disease by itself, but it is the manifestation of a wide spectrum of processes that may involve intraperitoneal or extraperitoneal organs. Transudative collections may be associated with portal hypertension, cirrhosis, heart failure, nephrotic syndrome or obstruction of the inferior vena cava, hepatic vein or portal vein. Exudative fluid may be related to infection or peritoneal carcinomatosis. Blood collections may be the result of trauma, haemorrhagic diathesis or tumour rupture. Bile collections may follow rupture of the biliary tree; chylous collections may develop after lymphatic obstruction; pancreatic fluid collections may be the consequence of acute pancreatitis; urine collections may represent extension of a retroperitoneal urinoma; purulent collections may be the consequence of visceral inflammation, intestinal perforation or surgery. In general, haematoma, biliary, urinary or purulent collections may be limited by active peritoneal reaction; such reactions form adhesions which limit collections, isolate inflammatory processes and may plug perforations. Consequently, exudates may not move freely in the peritoneal cavity and are usually located or isolated at the area where they develop compared with transudates, which diffuse throughout the peritoneal cavity with no significant peritoneal reaction. Peritoneal fluid moves along predictable pathways that are influenced by body habitus, gravity, intra-abdominal pressure gradients, adhesions and mesenteric reflections and attachments.3 At postmortem and at surgery ascites is mainly seen in the most dependent portion of the peritoneal cavity, namely the pouch of Douglas, which is the lowest and most posterior extension of the peritoneal reflections. In the intact abdomen, fluid migrates to the upper abdomen due to the lower hydrostatic pressure in the subdiaphragmatic related to respiratory movements. Fluid migration occurs along both the paracolic gutters and especially the right one, which is wider and deeper than the left, which in addition has an anatomical obstacle created by the phrenicocolic ligament at the level of the splenic flexure. Peritoneal fluid in the paracolic gutters is distinguished from retroperitoneal fluid by the preservation of the retroperitoneal fat posteriorly to the ascending or descending colon, provided there is not a complete ascending or descending mesocolon. Ascites in the upper abdomen often accumulates in the pouch of Morison (or hepatorenal space, the most depended portion of the peritoneal cavity in the upper abdomen), the subdiaphragmatic spaces and the perihepatic and perisplenic spaces. Any amount of fluid that may be found in the inframesocolic compartment of the abdomen tends to move towards the lower pelvis; in the right inframesocolic compartment ascites flows along the surface of the small-bowel mesentery to a pouch at the ileocaecal conjunction and in continuation to the pelvis, while in the left inframesocolic compartment the fluid is directed to the surface of the sigmoid mesocolon and, thereafter, to the pelvis. The CT attenuation values of ascites range from 0 to +30 HU. CT attenuation values are non-specific, although attenuation increases with increasing protein content as a general rule. Acute haemoperitoneum can be distinguished from other fluid collections by its high attenuation values (>30 HU), but lower values may be observed. In the presence of large ascites, the small-bowel loops are usually centrally positioned within the abdomen. However, in patients with very tense ascites, bowel loops can be displaced from the central position in the absence of intraperitoneal mass. Ascitic fluid under tension may result in extraperitoneal mass effect. Peritoneal fluid that becomes loculated due to benign or malignant adhesions may appear as a cystic lesion with mass effect. The most common location of free intraperitoneal air while the patient is in the supine position is anterior to the liver. CT is superior to plain radiographs in detecting minute quantities of pneumoperitoneum and negative window levels (lung settings) are very helpful in disclosing it. Free air demonstrated on CT can be distinguished from gas in the bowel because of its non-dependent location and lack of haustral or small-bowel folds. Perforation of the small bowel may be associated by inflammatory reaction in the mesentery in the form of streaky soft-tissue densities along with presence of extraluminal gas locally. Rotational anomalies of the small-bowel mesentery around the superior mesenteric artery occur when the normal process of fetal gut development is arrested. Intestinal malrotation in adults is generally asymptomatic. Reversal of the normal relationship between the superior mesenteric artery and vein, i.e. artery located to the right of vein, twisting of the mesentery around the artery and absence of the normal horizontal duodenum are characteristic findings.5 Internal hernias are formed by protrusion of a viscus through a peritoneal or mesenteric aperture. They include paraduodenal (53%), pericaecal (13%), foramen of Winslow (8%), transmesenteric and transmesocolic (8%), intersigmoid (6%) and retroanastomotic (5%) hernias. Internal hernias are often difficult to identify clinically. They are asymptomatic or cause symptoms ranging from intermittent and mild digestive complaints to acute intestinal obstruction. Imaging plays an important role in their diagnosis, with CT being the method of choice. Paraduodenal, traditionally the most common type of internal hernias, result from congenital abnormalities in mesenteric peritoneal fixation. They are three times more frequent on the left side than on the right. Left paraduodenal hernias develop through the Landzert’s fossa, which is present in approximately 2% of the population and is located at the duodenojejunal junction; the hernia sac lies posterior to the inferior mesenteric vessels. CT shows an abnormal cluster of dilated loops behind the stomach and pancreas, lateral to the duodenojejunal junction with anterior displacement of the stomach. Anterior displacement of the inferior mesenteric vein is a helpful sign.5,6 In right-sided paraduodenal hernias, bowel herniates through Waldeyer’s fossa, behind the superior mesenteric artery and inferior to third portion of duodenum. It occurs most frequently in cases of a non-rotated small intestine. Imaging findings include encapsulated small-bowel loops in the right mid-abdomen with anterior displacement of the right colic vein, looping of the small intestine around the superior mesenteric vessels and abnormal position of the superior mesenteric vein relative to the artery.5 Transmesenteric hernias are increasing in incidence. They are more likely than other hernias to develop volvulus. In children, transmesenteric hernias are the most common type of internal hernia, related to congenital mesenteric defects. In adults, they are usually related to previous surgery, especially Roux-en-Y anastomoses. On CT, a cluster of dilated loops lying adjacent to the abdominal wall, without overlying omental fat lateral to the colon which is displaced centrally, provides an important clue (Fig. 30-3). The mesenteric vascular pedicle is characteristically engorged, stretched and crowded.7 Lymphangioma is the most common mesenteric cystic lesion. Other mesenteric cysts like enteric duplication cyst, enteric cyst and mesothelial cyst are very uncommon. Lymphangioma represents a congenital malformation of the lymphatic vessels arising from the bowel. The typical imaging appearance is that of a large, thin-walled, single or multiloculated cystic mass, with contents of water-to-fat attenuation on CT (Fig. 30-4) and of high signal intensity on T2-weighted MR images. Enhancement of the cyst wall and septa is seen. It is frequently closely associated with the small bowel. US is helpful in demonstrating the internal septations of the cystic mass.8 Large mesenteric lymphangiomas can be differentiated from ascites by the presence of septa, compression on adjacent intestinal loops and lack of fluid in the dependent peritoneal recesses. Infective/inflammatory infection of the peritoneal cavity may be localised (abscess) or generalised (peritonitis). The CT appearance of a peritoneal abscess is variable, depending primarily on its age. In the earliest stages it may appear as a mass displaying attenuation values approximating those of soft tissue. As the process advances, the abscess undergoes liquefactive necrosis. A definable wall that may exhibit contrast enhancement and a nearly water attenuation centre are features of a mature abscess on CT. Accompanying findings include thickening or obliteration of adjacent fat planes and displacement of adjacent structures. Gas within a loculated fluid collection is highly suggestive of abscess, but is not pathognomonic, because a necrotic non-infected tumour or a mass that communicates with the bowel may also contain air. The CT features of abscesses may overlap with other pathological processes such as haematomas, bilomas, urinomas, necrotic tumours or pseudocysts. MRI is superior to CT in differentiating a haematoma from an abscess, but may miss a small amount of gas within an abscess. A percutaneous fine needle aspiration may reveal the nature of a fluid collection and is important for abscess diagnosis by aspirating pus. Peritonitis is characterised by a generalised collection of intraperitoneal fluid occurring secondary to bacterial, granulomatous or chemical causes. Although bacterial peritonitis may be primary, it usually results from an intraperitoneal abscess or rupture of a hollow viscus. CT features include ascites in association with peritoneal and mesenteric thickening. Gadolinium-enhanced MR images may show smooth peritoneal enhancement. Tuberculosis of the peritoneum is an uncommon manifestation of tuberculosis that can occur after rupture of a caseous lymph node, from direct GI tract involvement by the disease or by lymphatic or haematogenous spread. Common findings include the combination of free or loculated ascites, thickened strands with crowded vascular bundles within mesentery, smooth uniform thickening of the peritoneum and a smudged pattern of omental involvement infiltrated by small ill-defined soft tissue (Fig. 30-5).9 On CT, high-attenuation ascites (20–45 HU), reflecting its high protein content, may be seen. Lymphadenopathy is a common manifestation of abdominal tuberculosis and mesenteric nodes are frequently affected. Peripheral enhancement with central low attenuation on CT may be seen and corresponds histologically to peripheral highly vascular inflammatory reaction around central liquefaction or caseous necrosis.10 This appearance is suggestive but not pathognomonic of tuberculosis as low-attenuation mesenteric lymph nodes may also be seen with Whipple’s disease, necrotic metastases, infection with Mycobacterium avium-intracellulare, the cavitating mesenteric lymph node syndrome of coeliac disease and occasionally lymphoma.11 Hydatid disease is most commonly due to Echinococcus granulosus. Peritoneal hydatidosis is usually the result of traumatic or surgical rupture of hepatic hydatid disease and results in cystic, usually septated, thin-walled space-occupying lesions.12 CT is the method of choice in peritoneal seeding (Fig. 30-6). A calcifying rim is a suggestive feature. Ultrasound is useful for the detection of membranes, septa and hydatid sand within the cyst.
Imaging of The Peritoneum, Mesentery and Omentum
Anatomical Considerations
Introduction
Peritoneal Spaces
Supramesocolic Space
Inframesocolic Space
Peritoneal Reflections
The Mesenteries
The Omentum
Pathological Considerations
Ascites
Intraperitoneal Air
Developmental/Congenital Anomalies
Rotational Anomaly
Developmental Defects
Mesenteric Cysts
Infections–Inflammations
Tuberculosis
Hydatid Disease