Tumor predisposition syndromes represent a heterogeneous group of multiorgan disorders, with many having substantial central nervous system involvement. This article highlights the common and uncommon manifestations of these syndromic disorders, the underlying genetic pathways, and the imaging findings. Radiologists must be aware of the diagnostic criteria, optimal imaging techniques (both for diagnosis and surveillance), as well as the innumerable imaging manifestations of these syndromes. Multidisciplinary approach and teamwork are essential in managing these patients, with imaging having a central role as more of these patients get diagnosed earlier and survive longer.
Key points
- •
Cancer predisposition syndromes affecting the central nervous system represent a diverse subgroup of lesions, including intra-axial malignant brain tumors, extra-axial nerve sheath tumors, spinal cord tumors, vascular masses, pituitary tumors, and various hamartomas, with markedly different phenotypes both within and outside the neural axis.
- •
Advances in knowledge of the underlying genomics and cancer pathways has enhanced understanding of the tumor behavior, resulting in improved diagnostics, better imaging screening, and better surveillance strategies.
- •
Imaging serves a critical role in early diagnosis, screening, and long-term follow-up of these patients, requiring specialized whole-body protocols and surveillance techniques for optimal management.
Introduction
The tumor syndromes covered here include both inheritable and noninheritable cancer syndromes. These syndromes are more appropriately referred to as cancer predisposition syndromes, because of their complex cancer pathways, and how it is passed on from one generation to the next. Well-identified genetic mutations, which often characterize these syndromes, predispose to phenotypically distinct tumors throughout the body. The syndromes present a unique challenge to oncologists and radiologists owing to multiorgan involvement, aggressive nature of the tumors, and associated endocrine abnormalities. Recent genomic characterization of these syndromes has led to revision and reclassification of the established syndromes as well as development of targeted therapies.
Screening and diagnosis for these syndromes require a plethora of advanced laboratory tests, which include comprehensive genetic testing. Multimodality, multiorgan imaging plays a quintessential role in the screening, early diagnosis, surveillance, and management of these syndromes.
Whole-Body Imaging
In addition to targeted organ-specific protocols, dedicated whole-body imaging is becoming an indispensable tool in evaluating cancer syndromes. Occasionally, whole-body imaging refers to a combination of different modalities including ultrasonography (US), computed tomography (CT), and magnetic resonance (MR) imaging, done separately. Another emerging approach is single-modality whole-body scanning to evaluate local multiorgan tumors as well as distant metastatic disease. In this scenario, whole-body MR imaging has taken precedence over whole-body CT or PET/CT, because of lack of ionizing radiation (given that many of these patients are in the pediatric age group and need repeated follow-up scans), excellent soft tissue contrast, and bone marrow evaluation. Single-modality imaging also has the advantage of 1-time sedation or general anesthesia when required.
MR imaging has limitations, which include cost, decreased specificity, artifacts related to patient hardware and motion, repeated gadolinium dosage and accumulation, as well as technical issues with older scanners. However, recent advances have allowed many of the limitations to be overcome, which include rolling table platforms, multiple phased array coils, parallel imaging techniques, and wider field of view (FOV). These advances have led to improvement in resolution, decreased artifacts and scan time, with reduction in the need for repeated gadolinium administration. The principles of whole-body MR scans include using series of images at multiple stations (targeting specific body parts based on the FOV and patient size), in multiple planes (usually axial and coronal planes with sagittal plane used for spine), while using a combination of sequences tailored for specific disorders. The stations include combinations such as the chest-abdomen-pelvis station, neck-chest-abdomen-pelvis station, or brain–head and neck–spine station. Typically a combination of diffusion and fluid-sensitive sequences are used, with respiratory gating for chest and antiperistaltic drugs for abdomen. Use of gadolinium is reserved for specific syndromes and clinical situations.
Cancer predisposition syndromes
Neurofibromatosis
Neurofibromatosis is a group of hereditary syndromes with tumors involving the central and peripheral nervous systems, including neurofibromatosis type 1 (NF1), neurofibromatosis type 2 (NF2), and schwannomatosis.
Neurofibromatosis Type 1
NF1, also known as peripheral neurofibromatosis and von Recklinghausen disease, is an autosomal dominant (AD) syndrome, with high penetrance and variable expressivity, with an incidence of 1 in 3000. The mutation affects the neurofibromin tumor suppressor gene located in the long arm of chromosome 17 (17q11.2). About half are inherited and the rest result from sporadic mutations. The segmental form with a limited distribution of lesions can be seen with mosaicism. , Familial spinal neurofibromatosis is a rare form, with neurofibromas affecting nerve roots bilaterally at all spinal levels, with most patients lacking the cutaneous and ocular lesions of NF. The diagnostic criteria for NF1 are summarized in Box 1 .
Diagnostic criteria of NF1: 2 or more of the following criteria should be met:
- 1.
At least 6 café-au-lait macules (diameter>5 mm in prepubertal individuals and >15 mm in postpubertal individuals)
- 2.
Freckling in axillary or inguinal regions
- 3.
Optic glioma
- 4.
At least 2 Lisch nodules (iris hamartomas)
- 5.
At least 2 neurofibromas of any type, or 1 plexiform neurofibroma
- 6.
A distinctive osseous lesion (sphenoid dysplasia or tibial pseudarthrosis)
- 7.
A first-degree relative with NF1
- 1.
Neurofibromas
Neurofibromas, which are nerve sheath tumors, are the most common tumors in NF1, and can involve both superficial and deep soft tissues, including the nerve roots in the spinal canal and neural foramen. Morphologic subtypes include localized neurofibroma, plexiform neurofibroma (PNF), and diffuse neurofibroma.
Localized cutaneous neurofibroma is the most common form, presenting as nodular or polypoid lesion often with epidermal hyperpigmentation. Localized intraneural neurofibroma causes fusiform enlargement of the affected nerve. The lesions appear hypodense with minimal enhancement in CT scans, well defined and hyperintense on T2-weighted imaging (T2WI), with intermediate to low signal intensity on T1-weighted imaging (T1WI), with homogeneous, slightly heterogeneous, or central enhancement ( Figs. 1 and 2 ). The lesions may show target sign with low central signal with peripheral high signal in T2WI (see Fig. 2 ), and a central dense area on CT. Target sign can sometimes also be seen with schwannoma and malignant peripheral nerve tumors. Neurofibroma is challenging to differentiate from schwannoma by imaging. T2 hyperintense rim and intratumoral cysts are more common with schwannoma than with neurofibroma. ,


PNF is virtually pathognomonic of NF1, and appears as lobulated, tortuous thickening of nerves with a bag-of-worms appearance ( Fig. 3 ). The lesion appears as an infiltrative multispatial soft tissue density lesion in CT ( Fig. 4 ). Target sign is commonly seen (see Fig. 4 ). The lesion has intermediate signal intensity in T1-weighted images, with central dotlike or diffuse heterogeneous enhancement (see Figs. 3 and 4 ). ,


Diffuse neurofibromas most commonly occur in children and young adults as plaquelike or infiltrative, poorly defined, reticulated lesions in skin and subcutaneous fat.
Malignant peripheral nerve sheath tumor
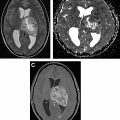
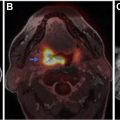
Malignant peripheral nerve sheath tumors (MPNSTs) usually arise from plexiform neurofibromas, and less commonly from localized neurofibromas. Malignant transformation of neurofibroma is often associated with rapid growth and worsening pain. Infiltrative margin, heterogeneous enhancement with necrotic areas, peripheral edema, and osseous destruction are features suggestive of malignant transformation ( Fig. 5 ). MPNST shows high 18 F-fluorodeoxyglucose (FDG) uptake on PET scans. About half of the MPNSTs occur in patients with NF1, and about 5% of patients with NF1 develop MPNST. ,

Optic pathway gliomas
NF1-associated optic pathway glioma (OPG) typically presents in young children. OPGs usually are low-grade pilocytic astrocytomas. Less than half of the cases are symptomatic. OPG in patients with NF1 commonly involves the optic nerve, followed by the chiasm, unlike OPG in patients without NF1. Hypothalamic involvement can present with premature or delayed puberty.
OPG appears as fusiform enlargement of the nerve that is isodense to slightly hypodense on CT scans. The optic canal may appear enlarged. Calcification is rare, which helps to differentiate OPG from optic sheath meningioma. OPG appears hyperintense on T2WI, and shows variable contrast enhancement ( Fig. 6 ). Cysts are uncommon in OPG associated with NF1. ,

Other central nervous system and extra–central nervous system tumors
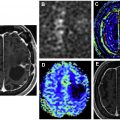
Brainstem gliomas are the second most common intracranial tumors in NF1 ( Fig. 7 ), frequently pilocytic astrocytomas with few transforming into aggressive gliomas ( Figs. 8 and 9 ). There is increased risk of rhabdomyosarcomas, gastrointestinal stromal tumors, endocrine tumors of gastrointestinal tract, pheochromocytomas, breast cancer, and hematopoietic malignancies.



Other neuroimaging findings
An unusual but characteristic imaging finding, known as focal abnormal signal intensity (FASI), can be seen in basal ganglia, thalamus, pons, midbrain, cerebellar peduncles, cerebellar white matter, dentate nuclei, and less commonly supratentorial white matter, likely from myelin vacuolization ( Fig. 10 ). These findings are usually hyperintense on T2WI, isointense on T1WI, with a few appearing mildly hyperintense on T1 in basal ganglia, likely because of microcalcifications. Although they typically have no associated mass effect or enhancement, rarely they show mild mass effect in basal ganglia. FASI can become bigger in early childhood before they regress. , Cerebrovascular dysplasia including stenosis, hypoplasia, moyamoya pattern ( Fig. 11 ), and aneurysms can occur.


Neurofibromatosis Type 2
Just like NF1, NF2 is also an AD genetic syndrome, with high penetrance and variable expressivity. However, it is rarer, with an incidence of 1 in 25,000. NF2 results from a mutation of the NF2 gene, which codes for the cytoskeletal protein schwannomin (merlin) located on chromosome 22 (22q12.2), which acts as a tumor suppressor protein. About half of the cases have de novo mutation, with somatic mosaicism in about one-third of these cases.
The diagnostic criteria for NF2 are summarized in Box 2 . The 1987 National Institute of Health (NIH) criteria were modified to include patients with multiple schwannomas and or meningiomas with no family history and who have not yet developed bilateral eighth nerve tumors. These criteria include the Manchester criteria, National Neurofibromatosis Foundation (NNFF) criteria, and Baser criteria ( Box 3 ). Young patients (<18 years old) with apparently isolated meningioma or vestibular schwannoma have a 20% and 10% likelihood, respectively, of developing NF2. After 20 years, likelihood decreases, and NF2 becomes very unlikely after 30 years. In contrast, 50% of patients more than 70 years of age with bilateral vestibular schwannoma without other features of NF2 represent a chance occurrence rather than underlying mosaic or constitutional NF2 mutation.
A patient who meets either condition A or B has NF2.
- A.
Bilateral vestibular schwannomas
- B.
First-degree family relative with NF2 and unilateral vestibular schwannoma or any 2 of neurofibroma, meningioma, glioma, schwannoma, or juvenile posterior subcapsular lenticular opacity
- A.
A patient who meets any 1 of the following criteria has NF2:
- 1.
Bilateral vestibular schwannomas if less than 70 years of age.
- 2.
First-degree relative with family history of NF2 (excluding siblings with clearly unaffected parents) and unilateral vestibular schwannoma if less than 70 years of age.
- 3.
First-degree relative with family history of NF2 or unilateral vestibular schwannoma and 2 of the following lesions: meningioma, ependymoma, schwannoma, cataract, cerebral calcification. LZTR1 (leucine-zipperlike transcription regulator 1) test has to be negative, if unilateral vestibular schwannoma plus ≥2 nonintradermal schwannomas.
- 4.
Multiple meningiomas (2 or more) and 2 of unilateral vestibular schwannoma, schwannoma, cataract, cerebral calcification.
- 5.
Constitutional pathogenic NF2 gene variant in blood or identical in 2 tumors.
- 1.
Schwannomas
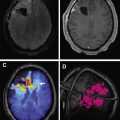
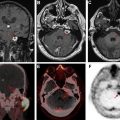
In addition to bilateral vestibular schwannomas ( Fig. 12 A, B) usually occurring by 30 years of age in about 90% to 95% of patients, schwannomas of other cranial nerves are present in up to about half of the patients ( Fig. 12 C). Unilateral hearing loss and tinnitus are the presenting symptoms in more than half of the adults and about a quarter of the children. Schwannomas can also affect spinal ( Fig. 13 ) and peripheral nerves. Vestibular schwannomas appear as enhancing masses that are ovoid when small, and ice cream cone–shaped when large enough to extend in to the cerebellopontine angle from the internal auditory canal (IAC). These lesions show up as filling defects within the cerebrospinal fluid (CSF) on high-resolution heavily T2-weighted sequences (see Fig. 12 ). CT scan shows widening of the IAC, whereas the tumor may be difficult to see on soft tissue windows.


Meningiomas
Intracranial meningiomas are present in about half of the patients, and spinal meningiomas are present in about 20%. Although many of these lesions remain asymptomatic, larger lesions, as well as smaller lesions within the spinal canal, optic canal, or skull base, can be symptomatic. They appear as extra-axial masses, usually with intense contrast enhancement (see Fig. 12 ; Fig. 14 ), similar to sporadic meningiomas.

Ependymomas
Ependymomas are present in up to about half of patients, mostly within the upper cervical canal or at the craniocervical junction. On imaging, these look similar to their sporadic counterparts, including showing hemosiderin cap and homogeneous enhancement (see Fig. 14 ).
Other central nervous system tumors
Intramedullary astrocytomas and schwannomas of the spinal cord and meningioangiomatosis have also been reported in NF2. ,
Schwannomatosis
Schwannomatosis is the third major form of neurofibromatosis, with an incidence of 1 in 60,000 to 70,000, characterized by multiple schwannomas without evidence of bilateral vestibular schwannomas. Inheritance is AD, with a sporadic predominance. The tumor suppressor genes identified are SMARCB1 (SWI/SNF-Related Matrix-associated Actin-dependent Regulator of Chromatin Subfamily B Member 1) and LZTR1 (leucine-zipperlike transcription regulator 1), located in the long arm of chromosome 22. The diagnostic criteria for schwannomatosis are summarized in Box 4 . The overlap in presentation and phenotype of schwannomatosis and NF2 makes the clinical differentiation difficult. On imaging, the schwannomas look identical to those in NF2. Spinal ependymomas are not present in schwannomatosis, which helps in differentiating from NF2.
A patient should meet the following criteria for a diagnosis of schwannomatosis:
- 1.
Anatomically distinct 2 nonintradermal schwannomas, 1 of which has to be histologically confirmed.
- 2.
No evidence of bilateral vestibular schwannomas on imaging or NF2 mutation.
- 3.
Presence of 1 pathologically confirmed schwannoma, unilateral vestibular schwannoma, or intracranial meningioma and schwannomatosis in a first-degree relative.
- 1.
von Hippel-Lindau Syndrome
von Hippel-Lindau (VHL) is an AD syndrome arising from mutations in the VHL tumor suppressor gene located in chromosome 3 (3p25-26). The mutation causes overexpression of hypoxia-inducible messenger RNAs, including vascular endothelial growth factor, which results in angiogenesis, and proliferation of endothelial cells and pericytes that are characteristic of VHL-related tumors. Hemangioblastoma (HB) and endolymphatic sac tumor (ELST) are the most common central nervous system (CNS) tumors in VHL, with rare reports of choroid plexus papilloma. The average age of presentation of VHL-related tumors is the third to fourth decade. Tumor combinations in VHL often overlap with other syndromes, such as NF1 and multiple endocrine neoplasia (MEN) and pose a diagnostic challenge. The recommended diagnostic criteria by the Danish VHL coordination group are summarized in Box 5 . The recommended NIH screening guidelines for VHL tumors are summarized in Table 1 .
| VHL diagnostic criteria: positive if individual satisfies criteria 1 or 2 or both | |
| Criterion 1 | At least 2 manifestations stated below |
| Criterion 2 | At least 1 manifestation stated below plus pathogenic mutation of VHL (or) at least 1 first-degree relative with VHL |
| VHL manifestations that satisfy criteria | |
| Retina | Hemangioblastoma |
| CNS | Hemangioblastoma of cerebellum, medulla oblongata, and/or spinal cord |
| Temporal bone | Endolymphatic sac tumor |
| Kidney | Renal cell carcinoma |
| Pancreas | Neuroendocrine tumor and/or multiple cysts |
| Adrenal gland/sympathetic chain | Pheochromocytoma, paraganglioma, and/or glomus tumor |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree