CHAPTER 6 Imaging Surveillance for Locally Recurrent Disease
Effective palliative, as well as curative, treatment is now available for patients with recurrent breast cancer. Optimal therapy is dependent on an accurate assessment of the location and extent of recurrent disease, which dramatically influences treatment decision making. Gene expression analysis has been used to identify patients at increased risk for distant recurrence.1,2 There is also the suggestion that expression profiling may be helpful in identifying patients at greater risk for local recurrence.3 This information can potentially be used to tailor the frequency and types of imaging modalities used to monitor these patients.
Most often, the imaging assessment of recurrent disease extent requires use of both anatomic modalities, such as mammography, ultrasound, CT, and MRI, and molecular or metabolic imaging, utilizing positron emission tomography (PET) or combination PET/CT. While fluorodeoxyglucose (FDG) PET is a highly accurate method for evaluating the whole body for recurrence (both local and distant), anatomic and molecular imaging should be viewed as complementary. Hathaway and associates demonstrated the value of combining PET with MRI nearly a decade ago.4
The superb spatial resolution of CT and MRI is of great value in defining the size, shape, and precise location of suspected metastases, whether locoregional or distant. However, anatomically based imaging modalities may be suboptimal in separating active tumor from treatment-related changes. FDG PET can be very helpful in this situation. Additionally, whole-body PET can often change a patient’s stage; confirmation of local recurrence only may direct the treating physician toward a more aggressive, curative-intent approach (e.g., surgery, localized radiation, or both). One study showed that FDG PET changed the clinical stage in 36% of patients and led to a change in therapy in 58%.5
As with any tumor, knowledge of the typical biologic behavior and predictable sites of recurrence is helpful in focusing attention on specific regions. In patients treated with mastectomy, axillary node dissection, and chemotherapy, the most frequent sites of locoregional recurrence are the chest wall and supraclavicular nodes.6 Remote sites may include the mediastinum, internal mammary nodes, and most commonly, the skeleton.7
LOCOREGIONAL
Locoregional recurrence may occur in up to 22% of patients undergoing breast conservation surgery and radiation.8 Breast cancer 10-year local recurrence rates in women with negative surgical margins who undergo lumpectomy and radiotherapy may be as low as 10% or less.9–12 Although overall recurrence rates are low, higher recurrence rates have been reported in select patient groups, such as those with high-grade ductal carcinoma in situ (DCIS).13 In addition, recurrence rates in younger women and in women who do not undergo radiation therapy have been reported as high as 35%.14
Serial surveillance mammography, as well as diagnostic mammography when there is clinical suspicion of breast recurrence, remains the cornerstone of the imaging approach to previously treated breast cancer patients (Figure 1). Complementary studies include breast ultrasound and MRI, as discussed in Chapter 3 (Figures 2 and 3). Cases presented in this chapter illustrate the spectrum of imaging manifestations of locally recurrent breast cancer.
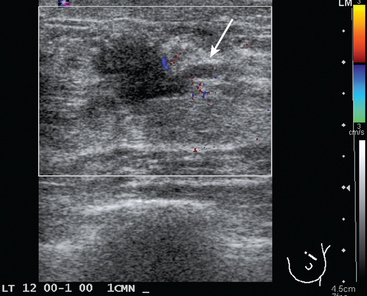
FIGURE 2 Ultrasound of the same patient in Figure 1 shows one of multiple solid masses identified by ultrasound. This mass has particularly suspicious sonographic features: it is solid and irregularly marginated with no definable capsule, and a tail of suspected ductal extension can be seen extending to the right (arrow).
Local failure most commonly occurs in or near the lumpectomy bed, in the same quadrant, and may manifest in a manner similar to the initial presentation (e.g., microcalcifications developing where calcified DCIS has previously been treated). Such changes arising in a lumpectomy bed under surveillance need careful analysis, such as with magnification views, to differentiate from similar-appearing, treatment-related changes (e.g., calcification due to fat necrosis). In some cases, differentiation based on morphology may not be possible, and tissue sampling will be required.
Palpable lumps developing in the breast, axilla, or chest wall of a patient previously treated for breast cancer raise the specter of recurrent disease, but not infrequently are due to treatment-related mimics, such as fat necrosis masses. Depending on location, these can be evaluated with some combination of mammography, ultrasound, and tissue sampling. At times, such mimics are sufficiently characteristic that imaging alone can make the diagnosis, but histologic sampling may be necessary for confirmation.
Mammographic follow-up is warranted in patients who have undergone subcutaneous mastectomy because of the considerable amount of remaining breast tissue. The yield from mammographic surveillance of the breast after standard mastectomy or skin-sparing mastectomy and reconstruction has been questioned. Because most recurrences occur in the skin and subcutaneous tissues, they can usually be detected on physical examination. The overall annual recurrence rate of T1 or T2 tumors following mastectomy has been reported to be 1% to 2% during the first 5 years.15 Annual mammographic evaluation following mastectomy and transverse rectus abdominis musculocutaneous (TRAM) flap reconstruction has been advocated to screen for any residual breast tissue and to detect recurrences before they are palpable.16
The timing for mammographic follow-up after breast conserving therapy has not been standardized. At a minimum, a 6-month follow-up mammogram should be performed to establish the patient’s baseline after surgery. The incidence of a recurrence in the ipsilateral breast 20 years after surgery is 14.3% among women who undergo irradiation after lumpectomy and 39.2% among those who undergo lumpectomy without irradiation.17
CHEST
Pathologic involvement of the internal mammary lymph nodes and mediastinal lymph nodes is best determined by PET.18 Although both areas are evaluable by CT, reliance on size alone has been shown to be inferior to assessment of metabolic activity in patients with non–small cell lung cancer (histologic gold standard).19 In one series of 73 patients, FDG PET was found to be twice as accurate as CT in identifying involved internal mammary or mediastinal nodes.20
BONE
Bone metastases are covered in depth in Chapter 8. Imaging options include plain radiographs, CT, MRI, nuclear medicine bone scans, and PET. Plain radiographs are readily available, are relatively inexpensive, expose the patient to a low level of radiation, require no patient preparation, and are completed rapidly. Typically, a minimum 30% reduction in bone mineralization is necessary before a radiograph becomes abnormal. Additionally, radiographs provide limited coverage. These factors reduce sensitivity both at the site of concern and for distant disease. Most commonly, bone radiographs are performed in an effort to improve the specificity of a nuclear bone scan. Even this function is being replaced by the utilization of CT or MRI for scintigraphic correlation. Both of these modalities dramatically improve diagnostic accuracy, compared with plain radiographs, in bone and surrounding soft tissue. However, the screening method of choice remains the nuclear medicine bone scan. It is a whole-body scan, delivers low radiation, and is exquisitely sensitive to changes in bone metabolism. PET and PET/CT utilizing F-18 FDG and sodium fluoride (NaF), are covered in greater detail in Chapter 8. Preliminary data suggest an advantage for PET over bone scintigraphy in the identification of skeletal metastases (especially lytic metastases). NaF, also a positron-emitting pharmaceutical, is a bone-specific agent, whereas FDG PET allows a whole-body (bone and soft tissue) evaluation.
DISTANT METASTASES
The confirmation and quantitation of distant metastases has both therapeutic and prognostic implications. Imagers are often asked to provide answers to the following questions: (1) Are distant metastases present? (2) Is the disease focal or widespread? (3) What are the prognostic implications? (4) In the absence of symptoms, with elevated serum tumor markers, where is the disease? Since the answers to questions 1 to 3 are applicable to most patients under investigation for recurrence, in many centers, PET has become the modality of choice to search for breast cancer metastases following initial therapy. Whole-body surveys have shown FDG PET to be both sensitive and specific for detection of distant disease.19–22
1 van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999-2009.
2 Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817-2826.
3 Mamounas E, Tang G, Bryant J, et al. Association between the 21-gene recurrence score assay (RS) and risk of locoregional failure in node-negative, ER-positive breast cancer: results from NSABP B-14 and NSABP B-20 [abstract]. Presented at the San Antonio Breast Cancer Symposium 28th Annual Meeting, December 10, 2005.
4 Hathaway PB, Mankoff DA, Maravilla KR, et al. The value of combined FDG-PET and magnetic resonance imaging in the evaluation of suspected recurrent local-regional breast cancer: preliminary experience. Radiology. 1998;210(3):807-814.
5 Yap CS, Seltzer MA, Schiepers C, et al. Impact of whole-body 18-FDG PET on staging and managing patients with breast cancer; the referring physician’s perspective. J Nucl Med. 2001;42(9):1334-1337.
6 Katz A, Strom EA, Bucholz TA, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000;18:2817-2827.
7 Eubank WB, Mankoff DA. Current and future uses of positron emission tomography in breast cancer imaging. Semin Nucl Med. 2004;34(3):224-240.
8 Huston TL, Simmons RM. Locally recurrent breast cancer after conservation therapy. Am J Surg. 2005;189(2):229-235.
9 Smitt MC, Nowels KW, Zdeblick MJ, et al. The importance of the lumpectomy surgical margin status in long-term results of breast conservation. Cancer. 1995;76:259-267.
10 Neuschatz AC, DiPetrillo T, Safaii H, et al. Long-term follow-up of a prospective policy of margin-directed radiation dose escalation in breast-conserving therapy. Cancer. 2003;97:30-39.
11 Obedian E, Haffty BG. Negative margin status improves local control in conservatively managed breast cancer patients. Cancer J Sci Am. 2000;6:28-33.
12 Provenzano E, Hopper JL, Giles GG, et al. Histological markers that predict clinical recurrence in ductal carcinoma in situ of the breast: an Australian population-based study. Pathology. 2004;36:221-229.
13 Borg MF. Breast-conserving therapy in young women with invasive carcinoma of the breast. Australas Radiol. 2004;48:376-382.
14 Scott WJ, Gobar LS, Terry JD, et al. Mediastinal lymph node staging of nonsmall cell lung cancer: a prospective comparison of computed tomography and positron emission tomography. J Thorac Cardiovasc Surg. 1996;111(3):642-648.
15 Kroll SS, Schusterman MA, Tadjalli HE, et al. Risk of recurrence after treatment of early breast cancer with skin-sparing mastectomy. Ann Surg Oncol. 1997;4:193-197.
16 Helvie MA, Bailey JE, Roubidoux MA, et al. Mammographic screening of TRAM flap breast reconstructions for detection of nonpalpable recurrent cancer. Radiology. 2002;224:211-216.
17 Fisher B, Anderson S, Bryant J, et al. Twenty-year follow up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233-1241.
18 Eubank WB, Mankoff DA, Takasugi J, et al. 18 Fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J Clin Oncol. 2001;19(15):3516-3523.
19 Lonneux M, Borbath L, Berliere M, et al. The place of whole-body PET/FDG for the diagnosis of distant recurrence of breast cancer. Clin Positron Imaging. 2000;3:45-49.
20 Hubner KF, Smith GT, Thie JA, et al. The potential of F-18-FDG/PET in breast cancer: detection of primary lesions, axillary lymph node metastases, or distant metastases. Clin Positron Imaging. 2000;3:197-205.
21 Gallowitsch HJ, Kresnik E, Gasser J, et al. F-18 fluorodeoxyglucose positron-emission tomography in the diagnosis of tumor recurrence and metastases in the follow-up of patients with breast carcinoma: a comparison to conventional imaging. Invest Radiol. 2003;38:250-256.
22 Kamel EM, Wyss MT, Fehr MK, et al. [18F]-fluorodeoxyglucose positron emission tomography in patients with suspected recurrence of breast cancer. J Cancer Res Clin Oncol. 2003;129(3):147-153.
CASE 1 Postoperative scar with hematoma on MRI
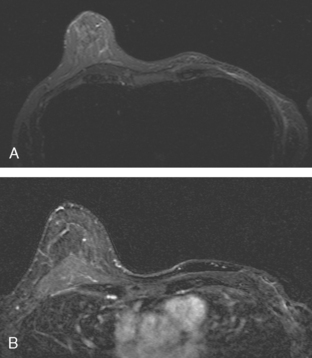
A 47-year-old woman with dense breasts was evaluated with a breast MRI 1 year after being treated for stage I, T1N0M0 right breast cancer. Her tumor was a 1.5-cm infiltrating ductal carcinoma (IDC) with mucinous and clear cell features, without angiolymphatic invasion, estrogen receptor and progesterone receptor positive, with margins clear for invasion and notable only for a focal close (1 mm) inferior medial margin for ductal carcinoma in situ (DCIS). Five sentinel lymph nodes were negative. She had been treated with partial mastectomy, four cycles of doxorubicin (Adriamycin) and cyclophosphamide (Cytoxan) (AC) chemotherapy, and radiation therapy, with a boost to the lumpectomy bed, and was taking tamoxifen. See, Case 8 in Chapter 1 for the imaging features of this patient’s breast cancer at initial diagnosis.
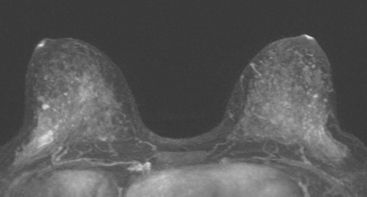
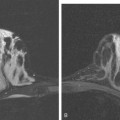
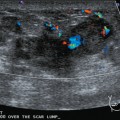
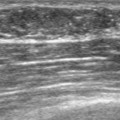
Because of her extreme breast density, surveillance breast MRI and ultrasound were obtained 1 year after breast cancer treatment. At the lumpectomy level, findings of a small residual hematoma were seen at the scar level on MRI (Figures 1, 2, and 3). The ultrasound correlate is seen in Figure 4.
TEACHING POINT
Most postoperative fluid collections are seromas, which display fluid signal on MRI (low on T1 and high signal on short tau inversion recovery (STIR) and T2). Hematomas may be differentiated from seromas by recognition of blood product signal intensity. In this example, bright signal seen on unenhanced T1-weighted sequences is methemoglobin, and the hypointense rim, best seen on gradient echo sequences, is characteristic of hemosiderin.
CASE 2 Changes from recent bilateral mastectomy and tissue expander placement
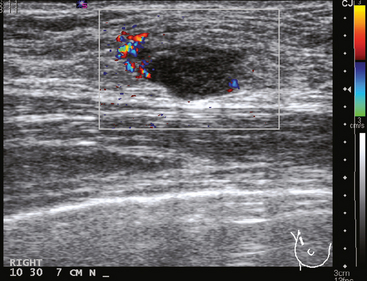
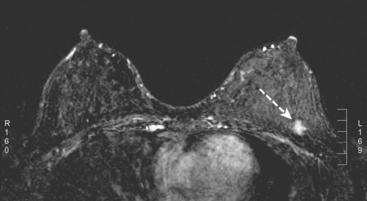
A 36-year-old woman noted a palpable right upper outer quadrant (UOQ) breast mass. Breast imaging evaluations showed a corresponding 1-cm hypoechoic, solid, vascular, sonographically indeterminate breast mass. Ultrasound-guided biopsy identified infiltrating ductal carcinoma (IDC) (Figure 1).
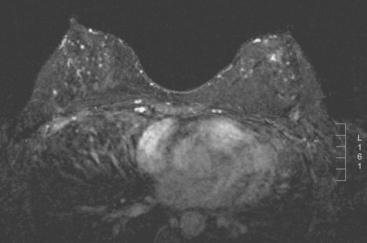
The past medical history was notable for prior mantle radiation for Hodgkin’s disease 12 years before. Preoperative breast MRI evaluation showed the known IDC as a 1.1-cm, early enhancing mass with washout, with a 4-mm suspected satellite nodule adjacent. A separate, lobular 5 × 3-mm possible intramammary lymph node was noted in the lateral breast. The left breast showed no focal or concerning findings (Figures 2 and 3).
The patient elected to undergo bilateral mastectomies, with tissue expanders placed. The right mastectomy specimen contained a 0.9-cm IDC, with high-grade ductal carcinoma in situ (DCIS) extending 5 mm beyond the invasive carcinoma, estrogen receptor negative, HER-2/neu negative. Atypical ductal hyperplasia was identified in multiple quadrants, as well as found in the left mastectomy specimen. The margins were negative. Four right axillary lymph nodes showed no evidence of disease.
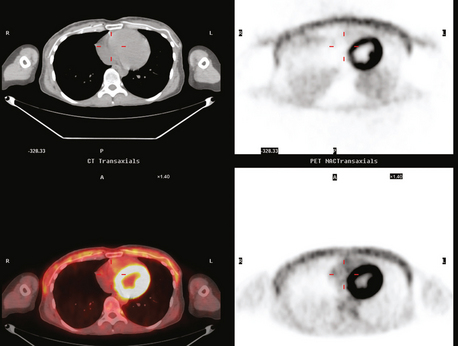
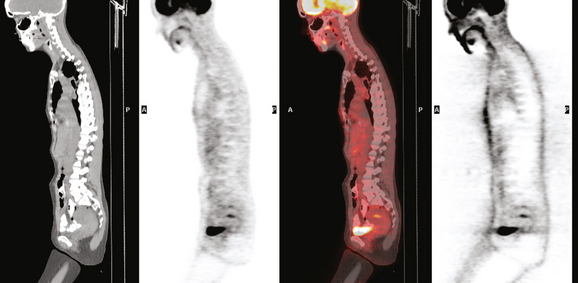
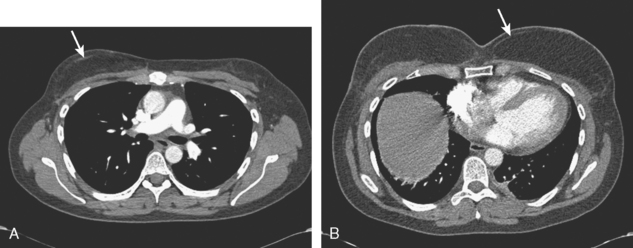
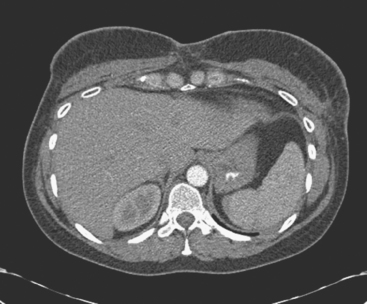
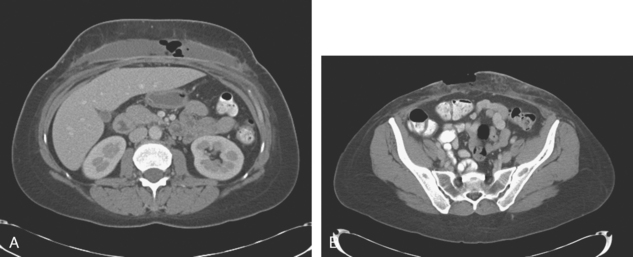
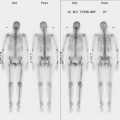
Staging evaluations with positron emission tomography (PET) and CT obtained before chemotherapy showed chest wall changes attributable to surgery, as well as normal variant endometrial activity (Figures 4, 5, and 6).
TEACHING POINTS
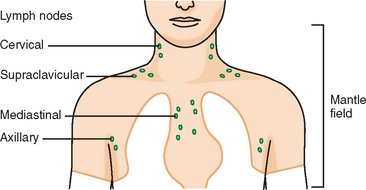
Younger patients treated for lymphoma with mantle radiation are at increased risk for a second malignancy (the incidence is 10% to 20% at 20 years of follow-up). Mantle radiation includes the neck, supraclavicular, infraclavicular, axillary, mediastinal, and hilar regions (Figure 7). The unshielded upper outer quadrants of the breasts receive the highest doses.
In women, if a second malignancy develops, it is most commonly breast cancer.
The normal, early postoperative findings of mastectomy with tissue expander placement are demonstrated here. A described, normal variant source of fluorodeoxyglucose (FDG) activity on whole-body PET is also seen here in the pelvis. Endometrial activity is most commonly seen in premenopausal patients during the first (menstruation) or third (ovulation) week of the menstrual cycle. If endometrial activity is identified on PET in a postmenopausal patient, this warrants further investigation, because endometrial carcinoma can manifest this way.
Estabrook A, Giron G. Treatment of unusual malignant neoplasias and clinical presentations. In: Roses DF, editor. Breast Cancer. 2nd ed. Philadelphia: Churchill Livingstone; 2005:705-706.
Lerman H, Metser U, Grisaru D, et al. Normal and abnormal 18F-FDG endometrial and ovarian uptake in pre- and postmenopausal patients: assessment by PET/CT. J Nucl Med. 2004;45:266-271.
Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290(4):465-475.
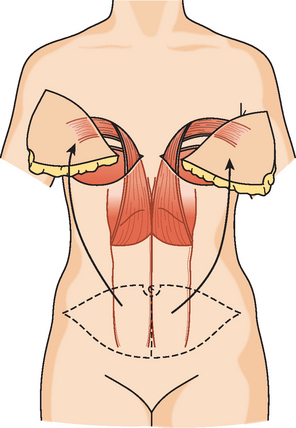
CASE 3 Normal CT appearance of bilateral TRAM flap reconstruction and post–TRAM flap abdominal complications
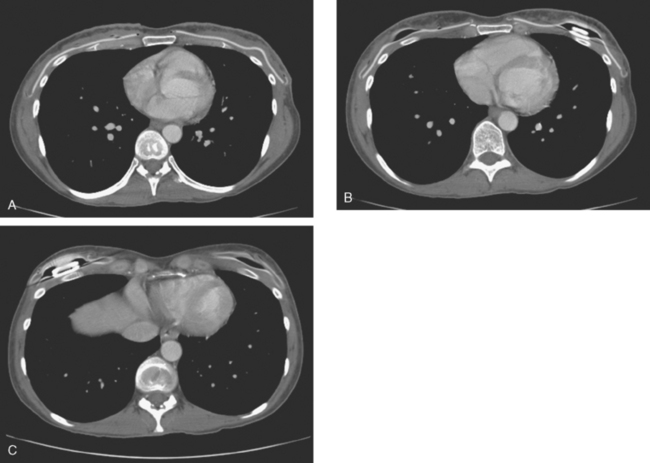
A 38-year-old woman elected to undergo prophylactic left mastectomy and bilateral transverse rectus abdominis musculocutaneous (TRAM) flap reconstruction 10 months after finishing radiation therapy for a right stage II, T2N1, 3-cm infiltrating ductal carcinoma (IDC), estrogen receptor and progesterone receptor negative, and HER-2/neu negative-, with 3 of 22 involved axillary lymph nodes. She had been treated with right mastec-tomy with clear margins, chemotherapy with four cycles of doxorubicin (Adriamycin) and cyclophosphamide (Cytoxan) (AC) and four cycles of paclitaxel (Taxol), and right chest wall, supraclavicular, and posterior axillary boost radiation therapy (see Case 18 in Chapter 3 for the presenting imaging features and initial staging of this patient).
The patient had undergone genetic testing subsequent to her diagnosis and treatment and proved to be BRCA-1 positive. Her family history was of premenopausal breast cancer in a maternal aunt. As a consequence, she decided to undergo hysterectomy and oophorectomy, which was performed at the same time as prophylactic left mastectomy and bilateral TRAM flap reconstruction (Figures 1, 2, and 3). Her left breast mastectomy specimen was benign.
The patient’s postoperative course was complicated by development of abdominal wound infection (Figure 4), requiring débridement twice of infected, necrotic tissue and intravenous antibiotic therapy, as well as anticoagulation for pulmonary embolism.
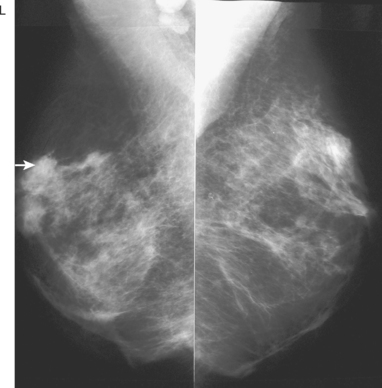
CASE 4 Recurrent DCIS presenting as new microcalcifications
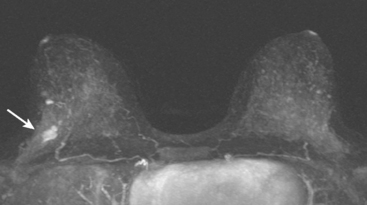
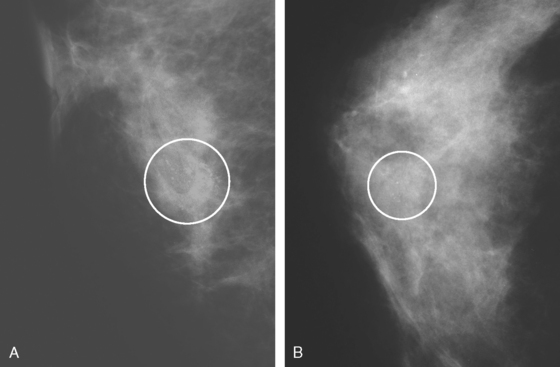
A 53-year-old woman, 2 years postmenopausal, underwent routine mammographic screening. A small cluster of faint, new, indeterminate microcalcifications was identified on the left (Figure 1), in the lower inner quadrant. The patient was unable to tolerate stereotactic biopsy and underwent surgical excision after needle localization. Pathology showed a 1-cm ductal carcinoma in situ (DCIS) lesion (with necrosis, cribriform, solid, and focal micropapillary forms), estrogen receptor negative, HER-2/neu positive, with a focal (0.2 cm) microinvasive component. The margins were close.
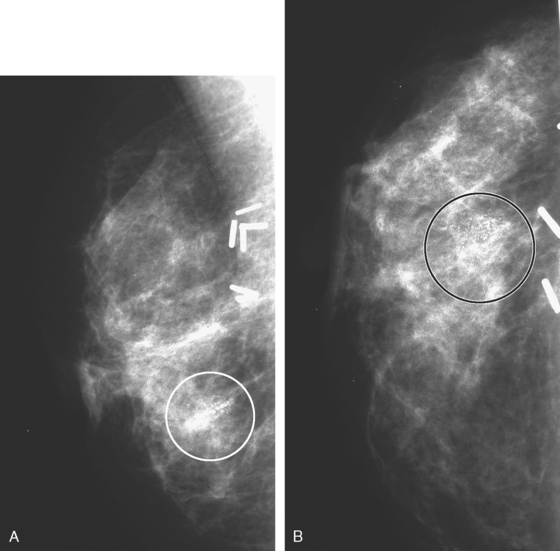
Eleven months after radiation therapy, routine mammographic surveillance showed new clustered left microcalcifications, which had developed since a prior study 6 months before (Figures 2 and 3). These were sampled stereotactically, and identified as DCIS.
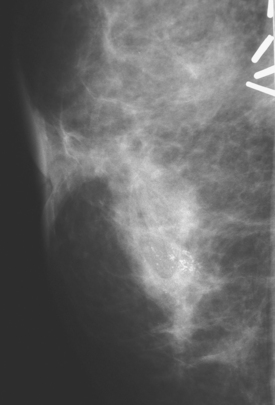
Mastectomy was performed. The histology showed a 6-mm focus of high-grade DCIS with comedo necrosis and negative margins. One examined axillary lymph node was negative. Breast MRI was obtained 1 month postoperatively to evaluate the opposite side, given the patient’s unusual clinical course (Figure 4). No concerning findings were seen.
Clinically, the patient was doing well 22 months after mastectomy.
TEACHING POINTS
This patient had an unusual clinical course, with rapid development of calcified DCIS only 11 months after undergoing treatment for DCIS with lumpectomy and radiation. In retrospect, perhaps breast MRI might have been useful in the initial evaluation of this patient’s extent of disease. The use of breast MRI with DCIS diagnoses, to look for invasive disease or to assess the extent of DCIS, is considered controversial and is far from universal in practice. However, because it is known that DCIS is often noncalcified, and not accurately mapped by mammography when noncalcified, and because DCIS on core biopsy is not infrequently upstaged on excision to micro or frank invasion, cogent arguments can be made to consider the use of breast MRI in initial staging of DCIS as well as invasive cancer.
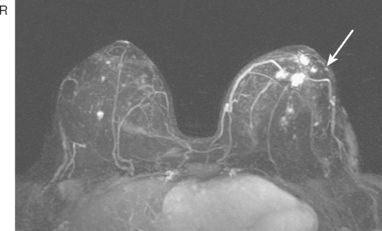
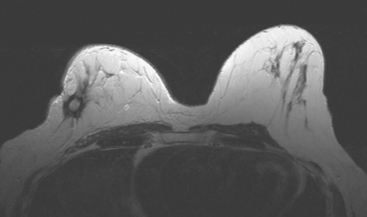
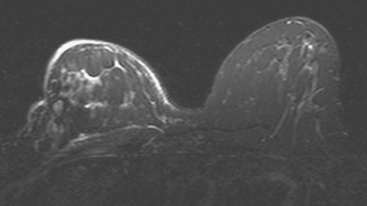
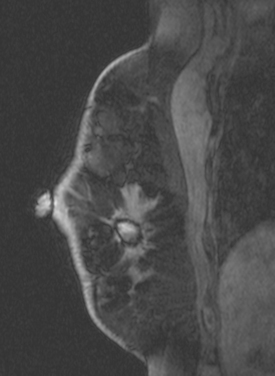
CASE 5 Recurrent DCIS detected on surveillance MRI
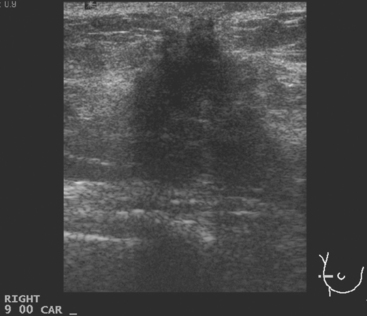
A 51-year-old woman, with prior left breast ductal carcinoma in situ (DCIS) treated with lumpectomy only, underwent annual screening MRI. The MRI demonstrated a developing 1.2-cm focal area of enhancement in the left breast, lateral to the patient’s prior lumpectomy site (Figures 1 and 2). Mammography showed no abnormality. Ultrasound demonstrated a small area of altered echotexture that appeared to correspond to the abnormal finding on MRI (Figure 3). Ultrasound-guided core needle biopsy identified intermediate-grade DCIS. The patient was treated with surgical excision and radiation therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree