7 Immune Disorders and Disorders of Uncertain Etiology Hypersensitivity reaction to Aspergillus spores with mucoid impaction. Most common bronchopulmonary mycosis in humans Type I or III hypersensitivity reaction in the bronchial system and the adjacent lung tissue CT is preferable to plain radiography. Linear shadow bands bifurcate to form V- or Y–shaped figures (bronchoceles, i.e., cystic mucus-filled bronchiectatic areas); there may be fluid shadows Bifurcating linear shadow bands Chronic recurrent asthma Steroids. Variable Suspected diagnosis.
Allergic Bronchopulmonary Aspergillosis
Definition
 Epidemiology
Epidemiology
 Affects younger adults (ages 20–40; women slightly more often than men)
Affects younger adults (ages 20–40; women slightly more often than men)  Atopic patients with bronchial asthma or in the setting of cystic fibrosis (occurs in 10% of cystic fibrosis patients, 1–2% of asthma patients).
Atopic patients with bronchial asthma or in the setting of cystic fibrosis (occurs in 10% of cystic fibrosis patients, 1–2% of asthma patients).
 Etiology, pathophysiology, pathogenesis
Etiology, pathophysiology, pathogenesis
 Leads to cystic-varicose bronchiectasis.
Leads to cystic-varicose bronchiectasis.
Imaging Signs
 Modality of choice
Modality of choice
 Radiographic and CT findings
Radiographic and CT findings
 Mucus obstruction causes atelectasis
Mucus obstruction causes atelectasis  Transient eosinophilic infiltrates
Transient eosinophilic infiltrates  Predilection for the proximal bronchial areas, and the upper and middle lung fields.
Predilection for the proximal bronchial areas, and the upper and middle lung fields.
 Pathognomonic findings
Pathognomonic findings
 “Finger in glove” and “toothpaste” shadows.
“Finger in glove” and “toothpaste” shadows.
Clinical Aspects
 Typical presentation
Typical presentation
 Cough with viscous sputum
Cough with viscous sputum  Hemoptysis
Hemoptysis  Eosinophilia
Eosinophilia  Hyphae in the sputum
Hyphae in the sputum  Antigen reaction.
Antigen reaction.
 Therapeutic options
Therapeutic options
 Course and prognosis
Course and prognosis
 Recurrent allergic bronchopulmonary aspergillosis leads to central bronchiectasis and fibrosis in the upper field.
Recurrent allergic bronchopulmonary aspergillosis leads to central bronchiectasis and fibrosis in the upper field.
 What does the clinician want to know?
What does the clinician want to know?
Differential Diagnosis
Cystic fibrosis and/or bronchiectasis | – No blood eosinophilia – Generalized changes and known underlying disorder in cystic fibrosis |
Bronchial atresia | – Usually in the apical posterior left upper lobe – Increased transradiancy and diminished vascularity – No history of allergy or cystic fibrosis |
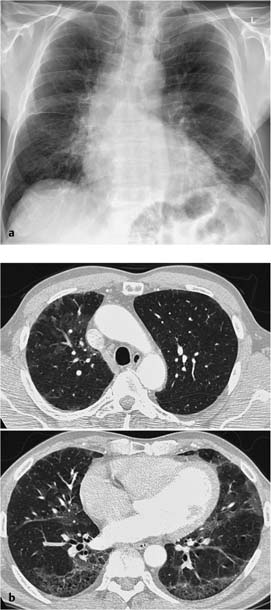
Fig. 7.1 Allergic bronchopulmonary aspergillosis in a 10-year-old girl with cystic fibrosis. The plain chest radiograph shows pronounced bronchial walls in the perihilar region and in the lower lung fields with faint bronchiectasis in the right middle lung field. Findings in the left upper lung field include an area of opacity that appears to “branch” peripherally. A similar, round pleural opacity is also seen nearby. These findings correspond to confluent areas of tubular impaction in allergic bronchopulmonary aspergillosis.
Bronchocentric granulomatosis | – Solitary or multiple sublobular consolidations in close proximity to the airways, usually unilaterally in the upper fields – Where asthma is present, findings are indistinguishable from allergic bronchopulmonary aspergillosis |
Tips and Pitfalls
Findings are nonspecific.
Selected References
Franquet T et al. Spectrum of pulmonary aspergillosis: histologic, clinical, and radiologic findings. Radiographics 2001; 21: 825–837
Johkoh T et al. Eosinophilic lung diseases: diagnostic accuracy of thin section CT in 111 patients. Radiology 2000; 216: 773–780
Ward S et al. Accuracy of CT in the diagnosis of allergic bronchopulmonary aspergillosis in asthmatic patients. AJR Am J Roentgenol 1999; 173: 937–942
Extrinsic Allergic Alveolitis or Hypersensitivity Pneumonitis
Definition
Immune reaction of the alveoli and bronchioles to inhalation of toxic substances  Synonym: Organic dust toxic syndrome.
Synonym: Organic dust toxic syndrome.
 Epidemiology
Epidemiology
Typical constellations are named after the specific type of exposure  Farmer’s lung (thermophilic actinomycetes)
Farmer’s lung (thermophilic actinomycetes)  Pigeon breeder’s lung (proteins)
Pigeon breeder’s lung (proteins)  Ventilator pneumonitis or Monday morning fever (heat tolerant bacteria), etc.
Ventilator pneumonitis or Monday morning fever (heat tolerant bacteria), etc.
 Etiology, pathophysiology, pathogenesis
Etiology, pathophysiology, pathogenesis
Allergic granulomatous reaction (bronchiolitis and alveolitis)  Inhalation of animal proteins, pathogenic microorganisms, spores, mineral oils, chemical organic substances (measuring 1–5 µm)
Inhalation of animal proteins, pathogenic microorganisms, spores, mineral oils, chemical organic substances (measuring 1–5 µm)  Prerequisite is an individual predisposition
Prerequisite is an individual predisposition  In the acute stage there is bronchiolitis with proliferation of alveolar cells
In the acute stage there is bronchiolitis with proliferation of alveolar cells  Subacute stage involves development of interstitial granulomas
Subacute stage involves development of interstitial granulomas  The chronic stage includes development of interstitial fibrosis, honeycomb lung, and cysts.
The chronic stage includes development of interstitial fibrosis, honeycomb lung, and cysts.
Imaging Signs
 Modality of choice
Modality of choice
CT.
 Radiographic findings
Radiographic findings
Findings are usually normal in the acute and subacute stages  Rarely, there will be discrete ground-glass opacities or nodular and reticulonodular changes
Rarely, there will be discrete ground-glass opacities or nodular and reticulonodular changes  The chronic stage includes fibrosis predominantly in the middle field that spares the costophrenic angle.
The chronic stage includes fibrosis predominantly in the middle field that spares the costophrenic angle.
 CT findings
CT findings
– Acute and subacute stages: Disseminated, ill-defined centrilobular nodules that can resemble ground-glass lesions  Diffuse or nodular ground-glass opacity
Diffuse or nodular ground-glass opacity  Predominantly in the middle and lower lung fields.
Predominantly in the middle and lower lung fields.
– Chronic stage: Honeycomb fibrosis, cystic changes, traction bronchiectasis, and volume loss  No pleural or subpleural involvement
No pleural or subpleural involvement  No lymphadenopathy.
No lymphadenopathy.
 Pathognomonic findings
Pathognomonic findings
In the subacute phase these include diffuse and nodular ground-glass opacities, ill-defined centrilobular nodules (acinar nodules), predominantly in the middle and lower lung fields  Later findings include fibrotic changes, acinar nodules, and ground-glass opacities.
Later findings include fibrotic changes, acinar nodules, and ground-glass opacities.
Clinical Aspects
 Typical presentation
Typical presentation
The acute stage (4–8 hours after exposure) is characterized by flulike symptoms, nonproductive cough with dyspnea, resolving within 1–2 weeks  The subacute chronic stage manifests as recurrent, episodic, and progressive shortness of breath (a sign of restrictive and obstructive pulmonary dysfunction).
The subacute chronic stage manifests as recurrent, episodic, and progressive shortness of breath (a sign of restrictive and obstructive pulmonary dysfunction).
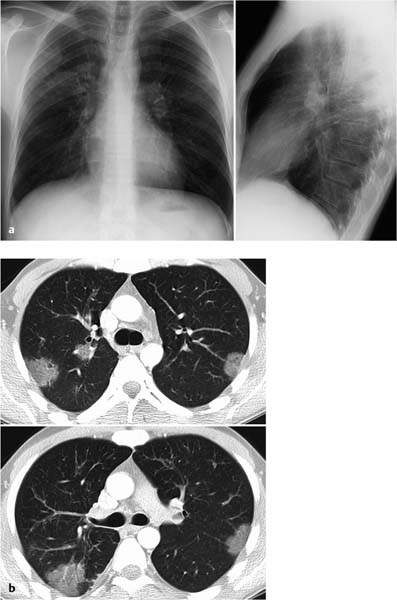
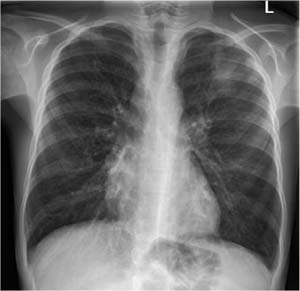
Fig. 7.2 Acute stage of extrinsic allergic alveolitis in a 45-year-old woman.
a The plain chest radiograph shows no abnormal findings.
b CT shows ground-glass opacification uniformly involving all lung segments.
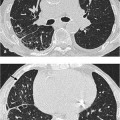
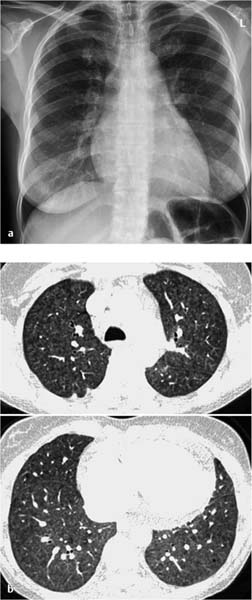
Fig. 7.3 Severe fibrosis of the lung within the framework of an extrinsic allergic alveolitis in a 60-year-old man (bird-keeper). Fibrosis with cystic parenchym alterations. Traction bronchiectasy, widening of the central tracheobronchial system and decreased lung volume, encapsulated pneumothorax on the right.
History (antigen exposure and time-dependent symptoms), specifically immunoglobulin G (IgG) antibodies, radiographic findings, decreased pO2  Diagnosis is confirmed by bronchoalveolar lavage and by bronchial provocation testing where indicated.
Diagnosis is confirmed by bronchoalveolar lavage and by bronchial provocation testing where indicated.
 Therapeutic options
Therapeutic options
Prophylaxis against exposure  Steroids.
Steroids.
 Course and prognosis
Course and prognosis
Variable  Prognosis is good with prompt diagnosis and prophylaxis against exposure.
Prognosis is good with prompt diagnosis and prophylaxis against exposure.
 What does the clinician want to know?
What does the clinician want to know?
Extent of organ manifestation  Disease activity
Disease activity  Course under therapy.
Course under therapy.
Differential Diagnosis
Toxic alveolitis | – Normal radiographic findings – No pulmonary dysfunction or impaired diffusion – No antibodies |
Bronchopulmonary infection | – History – Course under antibiotics – Bronchoalveolar lavage |
Nonspecific interstitial pneumonitis | – Ground-glass opacities – Peribronchovascular distribution – No honeycombing – Histologic examination recommended to exclude extrinsic allergic alveolitis |
Respiratory bronchiolitis with interstitial lung disease | – Smoker – Centrilobular emphysema – Primarily in the upper lobes |
Silicosis | – History of occupational exposure |
Sarcoidosis | – Peribronchovascular distribution – Lymphadenopathy |
Scleroderma | – Predominantly basal fibrosis – Esophageal dilatation |
Tips and Pitfalls
The acute stage is often misdiagnosed as a flulike infection  The chronic stage is indistinguishable from other chronic inflammatory pulmonary disorders.
The chronic stage is indistinguishable from other chronic inflammatory pulmonary disorders.
Selected References
Lynch DA et al. Can CT distinguish hypersensitivity pneumonitis from idiopathic pulmonary fibrosis? AJR Am J Roentgenol 1995; 165: 807–811
Matar LD, McAdams HP, Sporn TA. Hypersensitivity pneumonitis. AJR Am J Roentgenol 2000; 174: 1061–1066
Sennekamp J, Müller-Wening D. [Exogen-allergische Alveolitis.] Pneumologe 2006; 3: 461–470 [In German]
Eosinophilic Lung Disease—Eosinophilic Pneumonia
Definition
A group of etiologically and clinically heterogeneous disorders with tissue eosinophilia and usually but not invariably blood eosinophilia—Loeffler infiltrate  Acute eosinophilic pneumonia
Acute eosinophilic pneumonia  Chronic eosinophilic pneumonia
Chronic eosinophilic pneumonia  Idiopathic hypereosinophilic syndrome.
Idiopathic hypereosinophilic syndrome.
 Epidemiology
Epidemiology
Chronic eosinophilic pneumonia is often associated with atopic disorders (especially asthma)  Women are affected twice as often as men
Women are affected twice as often as men  Peak occurrence at age 30–40 years
Peak occurrence at age 30–40 years  Hypereosinophilia syndrome is more common in men than women by a ratio of 7: 1.
Hypereosinophilia syndrome is more common in men than women by a ratio of 7: 1.
 Etiology, pathophysiology, pathogenesis
Etiology, pathophysiology, pathogenesis
Etiology is either unclear or reactive secondary to drugs, parasites (ascarids, amebas), fungi, etc.  Morphologic findings include edema, and alveolar and interstitial eosinophilic infiltrates.
Morphologic findings include edema, and alveolar and interstitial eosinophilic infiltrates.
Imaging Signs
 Modality of choice
Modality of choice
CT is usually preferable to plain radiography.
 Radiographic and CT findings
Radiographic and CT findings
– Loeffler syndrome: Ill-defined transient or migrating nonsegmental infiltrates CT shows ground-glass or denser foci with halos.
– Acute eosinophilic pneumonia: Bilateral reticular and nodular nonsegmental opacities  CT shows ground-glass opacities.
CT shows ground-glass opacities.
– Chronic eosinophilic pneumonia: Homogeneous peripheral subpleural shadows (“negative image” of the edema) persisting for months, bilateral in 50% of cases, nonsegmental, predilection for the upper lobe, air bronchogram may be present  Resolves leaving bandlike densities parallel to the pleura
Resolves leaving bandlike densities parallel to the pleura  CT allows better evaluation of the characteristic topology.
CT allows better evaluation of the characteristic topology.
– Idiopathic hypereosinophilic syndrome: Loeffler-like picturebut persistent, due at least partially to cardiac involvement  Pleural effusion occurs in 50% of cases.
Pleural effusion occurs in 50% of cases.
 Pathognomonic findings
Pathognomonic findings
– Acute eosinophilic pneumonia: Edemalike infiltrates.
– Chronic eosinophilic pneumonia: Peripheral subpleural shadows (“negative image” of the edema) which resolve leaving bandlike densities parallel to the pleura.
– Loeffler syndrome: Transient infiltrates with halo.
– Idiopathic hypereosinophilic syndrome: Nodular infiltrates with halo.
Clinical Aspects
 Typical presentation
Typical presentation
– Loeffler syndrome: Minimally symptomatic.
– Acute eosinophilic pneumonia: Fever  Dyspnea
Dyspnea  Myalgia
Myalgia  Malaise
Malaise  Blood eosinophilia is not invariably present.
Blood eosinophilia is not invariably present.
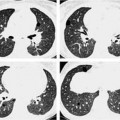
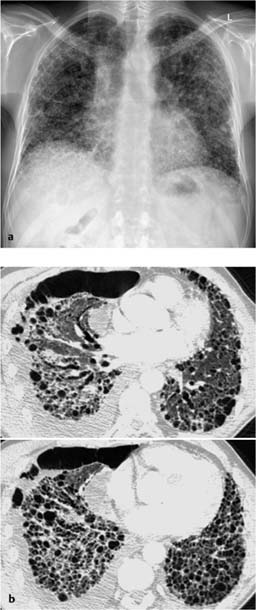
Fig. 7.4 Eosinophilic pneumonia in a 54-year-old man.
a The plain chest radiograph shows partially fine nodular and partially streaky reticular shadowing bilaterally and primarily in the basal region.
b Similar changes seen on CT, except that the nodular opacities appear as focal ground-glass opacities.
Fig. 7.5 Eosinophilic pneumonia in a 34-year-old man. The plain chest radiographs and CT show patchy, minimally dense infiltrates with pleural involvement in both upper lobes.
– Chronic eosinophilic pneumonia: Fever  Dyspnea
Dyspnea  Cough
Cough  Asthma is present in 50% of cases.
Asthma is present in 50% of cases.
– Idiopathic hypereosinophilic syndrome: Long history  Severe eosinophilia
Severe eosinophilia  Involves multiple organ systems (heart more than lung).
Involves multiple organ systems (heart more than lung).
 Therapeutic options
Therapeutic options
Simple pulmonary eosinophilia usually resolves spontaneously  Corticosteroids are indicated in the other idiopathic eosinophilic disorders.
Corticosteroids are indicated in the other idiopathic eosinophilic disorders.
 Course and prognosis
Course and prognosis
Simple pulmonary eosinophilia has a good prognosis even without treatment  Acute and chronic eosinophilic pneumonia and hypereosinophilic syndrome have a good prognosis with steroid therapy
Acute and chronic eosinophilic pneumonia and hypereosinophilic syndrome have a good prognosis with steroid therapy  Recurrence is rare in acute eosinophilic pneumonia but common in chronic eosinophilic pneumonia.
Recurrence is rare in acute eosinophilic pneumonia but common in chronic eosinophilic pneumonia.
 What does the clinician want to know?
What does the clinician want to know?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Diagnostic criteria
Diagnostic criteria