Incidental pancreatic cysts are commonly encountered in radiology practice. Although some of these are benign, mucinous varieties have a potential to undergo malignant transformation. Characterization of some incidental pancreatic cysts based on imaging alone is limited, and given that some pancreatic cysts have a malignant potential, various societies have created guidelines for the management and follow-up of incidental pancreatic cysts. This article reviews the imaging findings and work-up of pancreatic cysts and gives an overview of the societal guidelines for the management and follow-up of incidental pancreatic cysts.
Key points
- •
Incidental pancreatic cysts are commonly encountered in a radiology practice.
- •
Although some of these are benign, mucinous cystic lesions have a potential to undergo malignant transformation.
- •
Characterization of some incidental pancreatic cysts based on imaging alone is limited, and given that some pancreatic cysts have a malignant potential, guidelines exist to help determine management and follow-up based on current evidence and consensus agreements.
Introduction
Incidental pancreatic cysts (PCs) are commonly encountered in radiology practice. The prevalence rate of PCs is estimated at 2.5%. There is a 9% reported incidence on computed tomography (CT) and a 27% incidence on MR imaging. PCs are a heterogeneous group, including intraductal papillary mucinous neoplasm (IPMN), serous cystic neoplasm (SCN), and mucinous cystic neoplasm (MCN). Non-neoplastic PCs are pancreatic pseudocysts (common), epithelial cysts (uncommon), and lymphoepithelial cysts (rare). The significance in categorizing PCs lies in the potential of the mucinous varieties to develop malignancy. There is substantial variability in the malignant potential of the incidentally detected PC, particularly if they are too small or otherwise cannot be fully characterized by imaging. For this reason, multiple societies have published follow-up imaging guidelines and management plans aimed at detecting early malignant transformation. The guidelines have been complicated with concerns of imaging costs and over-screening. The prevalence rate of pancreatic ductal adenocarcinomas (PDACs) arising in patients with PCs is very low, at 33.2 per 100000; the rate of malignant transformation increases linearly with age. This review provides a practical understanding of PCs because they are commonly encountered in radiology practice. The radiologist has an important opportunity to work with pancreatic surgeons and gastroenterologists to provide optimal multidisciplinary care to the many patients with PCs.
Imaging technique and protocol
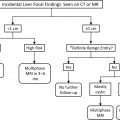
The American College of Radiology (ACR) Appropriateness Criteria for initial evaluation of an incidental PC without high-risk stigmata list MR imaging of the abdomen without and with contrast with MR cholangiopancreatography (MRCP) as “usually appropriate” and intravenous (IV) contrast-enhanced CT (CECT) as “may be appropriate”; cyst size cutoff of greater than 2.5 cm adds a recommendation for endoscopic ultrasound (EUS). For initial evaluation of an incidental PC greater than 2.5 cm, with worrisome features or high-risk stigmata, EUS and MR imaging of the abdomen without and with IV contrast with MRCP fall into the usually appropriate categories.
For an incidentally detected main pancreatic duct (MPD) dilated beyond 7 mm and suspicion for main duct (MD)-IPMN, EUS, and MR of the abdomen without and with IV contrast with MRCP are considered usually appropriate.
Computed Tomography Protocol
Pancreatic CT is performed in the pancreatic parenchymal and portal venous phases. The pancreatic phase represents peak pancreatic parenchymal enhancement, which occurs 40 seconds to 45 seconds following the IV injection of contrast. The portal venous phase is obtained 70 seconds to 75 seconds following the IV injection of contrast. Because the time of peak parenchymal enhancement can vary based on a patient’s physiology, an accurate method for achieving this phase entails triggering imaging at 16 seconds following the acquisition of a threshold of 175 Hounsfield units in the upper abdominal aorta. At UC Davis, the pancreatic CT protocol involves injecting 125 mL of iodinated contrast (iohexol, 350 mg Iodine/mL) at an injection rate of 4 mL/s. This acquisition approach also yields a late arterial phase which can be used for surgical resection planning of PDAC. One set of axial reconstructions is obtained at a slice thickness of 1.0 mm to 1.5 mm, which allows for better evaluation of small mural nodules, side duct branches, and for creating a curved-planar reformation (CPR) along the path of the main pancreatic duct. CPRs can better depict communication of a cyst with the pancreatic ductal system, changes in duct caliber, or intraductal enhancing masses.
MR Protocol
The pancreatic MR imaging protocol consists of multiplanar T2-weighted images, MRCP images, and precontrast and postcontrast 3-dimensional (3-D) –T1-weighted images. Coronal and axial single-shot fast spin-echo T2-weighted images with and/or without fat suppression are acquired because they allow for an anatomic overview and delineation of PCs. Fast spin-echo T2-weighted images are optional. Steady-state free-precession images provide contrast determined by the ratio of T2/T1. Fluid is high signal intensity and can be used as an alternative to single-shot fast spin-echo imaging. MRCP images can be acquired as 2-dimensional, thick (40 mm), heavily T2-weighted slabs and/or high-resolution (1–2 mm) 3-D volumetric acquisitions. The latter can result in superior assessment of the features of PCs and should be acquired routinely. Diffusion-weighted imaging now is a routine part of abdominal MR imaging, although not specifically necessary for PC follow-up.
Nonenhanced T1-weighted MR images are acquired using 3-D fat-suppressed spoiled gradient-echo sequences. The use of IV gadolinium in the follow-up of PCs is controversial. Studies have shown the addition of contrast adds little value in the follow-up of PCs and rarely changes management. , Nonenhanced MR imaging is faster, entails review of fewer images, and avoids the risks of IV gadolinium, although rarely IV contrast may be helpful in identifying high-risk features. One approach is to selectively administer contrast for larger cysts, cysts with known high-risk features, or for the initial evaluation of cysts, leaving nonenhanced MR imaging for follow-up of cysts known to be small and without high-risk features.
Ultrasound
Ultrasound (US) of the pancreas has been limited by the pancreas’ anatomic location as a retroperitoneal organ with overlying bowel. The utility of transabdominal US to assess PCs is inversely related to a patient’s weight and abdominal diameter.
Conventional US has a sensitivity of 94% for the differentiation of pseudocysts and cystic neoplasms but a relatively poor specificity, at 44%. The use of IV US contrast agents can increase the specificity to 97%, by demonstrating perfusion to small nodules or septations. IV contrast significantly improves the area under the curve in receiver operating characteristic curve analysis but has not yet gained widespread use.
Endoscopic ultrasound
EUS utilizes endoscopy to access the upper digestive tract. The endoscope is equipped with a small US transducer with high frequency and corresponding high spatial resolution ( Fig. 1 ). EUS has been shown to help differentiate pseudocysts from cystic neoplasms and guides fine-needle aspiration (FNA). SCNs have a heterogeneous appearance on EUS. Mural nodules, thick cyst walls, or intracystic growth are found more commonly in mucinous neoplasms. , EUS has been shown to be very sensitive in the diagnosis of PDAC during the follow-up of IPMNs, outperforming MR imaging, CT, and US. Variables most predictive of malignancy on EUS include mural nodules and MPD greater than or equal to 10 mm. The interobserver agreement of EUS is low for differentiating neoplastic versus non-neoplastic, type of PC, and EUS features of a PC; EUS is limited in the differentiation of mucinous from nonmucinous cysts. , US contrast increases the accuracy of detection of mural nodules in branch duct (BD)-IPMNs from 72% to 98%.

Approach to the incidental pancreatic cyst
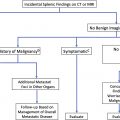
It is critical for radiologists to know if the PC actually is incidental. Any worrisome symptoms, such as pain, jaundice, or mass effect, should encourage further work-up, although PC signs and symptoms often are nonspecific. Clinical history, including patient age and gender as well as any known syndromes, may help in characterization.
Definitely benign
Serous Cystic Neoplasms
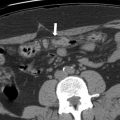
SCNs typically are described as having a honeycomb or multilocular appearance with or without a central scar. However, there can be variations in the morphologic appearance with polycystic, oligocystic, and solid patterns described , ( Figs. 2 and 3 ). Microcystic morphology is more common in SCNs ( Fig. 4 ). The classic imaging features are a lobulated external contour and central scar with stellate calcification ( Fig. 5 ). SCNs rarely demonstrate peripheral enhancing capsule or mural nodules.
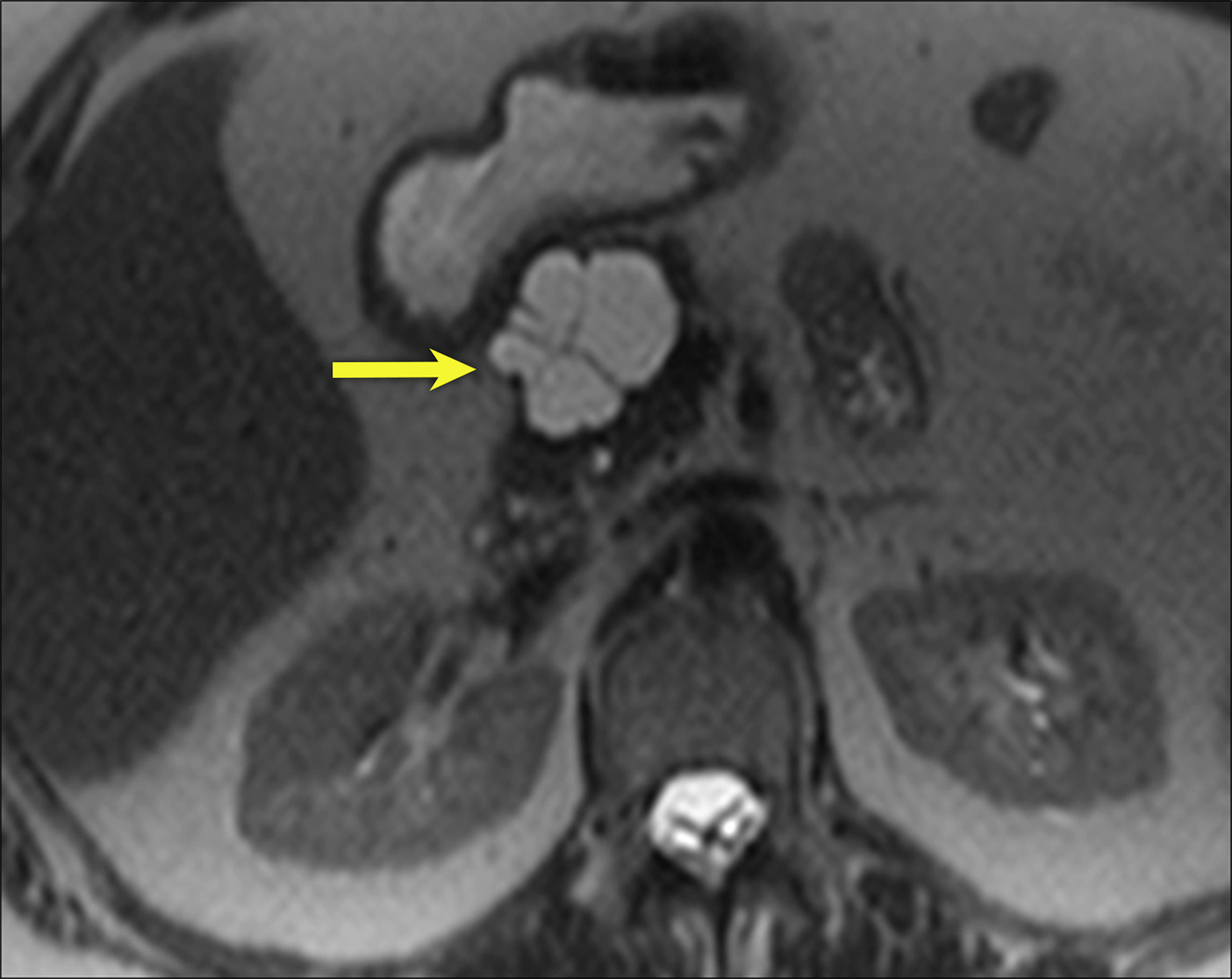



The combination of morphologic features, such as location in the body or tail, size, and lobulated contour plus textural analysis, yields a high area under the receiver operator characteristic curve in differentiating SCNs from MCNs. SCNs typically are isolated; however, patients with von Hippel-Lindau (VHL) disease may demonstrate multiple pancreatic masses in addition to cysts and tumors in other organ systems.
Pancreatitis-Associated Fluid Collections
Pancreatitis is an inflammatory condition of the pancreas, which can result in fluid collections with a cystic appearance. The revised Atlanta classification for acute pancreatitis describes acute peripancreatic fluid collections ( APFCs ) as fluid collections associated with pancreatitis in the first 4 weeks of inflammation, with acute necrotic fluid collections (although the term is still used loosely in practice); used to denote any associated necrosis. Pseudocyst is restricted to evolving peripancreatic fluid collections, although the term is used loosely in practice. If an APFC persists beyond 4 weeks and develops an enhancing capsule the term pseudocyst is used ( Fig. 6 ). If an acute necrotic collection lasts beyond 4 weeks and develops an enhancing capsule the term walled off necrosis is used. It is important for the radiologist to consider pseudocyst in the differential given the high prevalence of pancreatitis.

Congenital or Syndromic Pancreatic Cysts
Congenital PCs with an epithelial lining are rare. Pancreatic cystosis is a rare finding in cystic fibrosis in which the entire pancreatic parenchyma is replaced with macrocysts.
Lymphoepithelial Cyst
Lymphoepithelial cysts are rare cysts lined with squamous epithelium and surrounded by lymphoid tissue. They typically affect men who are middle-aged or older and are exophytic with a higher CT attenuation compared with SCNs and MCNs. The reference standard for diagnosis is excision.
von Hippel-Lindau Disease
VHL disease is an autosomal dominant disorder with tumors affecting multiple organ systems. The pancreas is affected in VHL disease by PCs, endocrine tumors, and SCNs ( Fig. 7 ).

Potentially or definitely malignant
Intraductal Papillary Mucinous Neoplasm
IPMNs are cystic neoplasms with variable degree of malignant potential. They may evolve into dysplasia or invasive carcinoma and are associated with a higher risk for the development of PDAC in the gland separate from the IPMN sites. The rate of progression increases with time. Low-risk IPMNs have an approximately 8% chance of progression, whereas higher risk IPMNs have an approximately 25% chance of progression to PDAC in 10 years. Even presumed low-risk BD-IPMNs may demonstrate growth after 5 years.
IPMNs may be separated into BD-IPMNs, with a clear connection to the main duct; MD-IPMNs, in which there is either focal or diffuse ductal dilatation; or mixed types ( Figs. 8–10 ). Filling defects are worrisome for malignancy. Variable MPD cutoff levels exist in the literature, with MPD dilatation between 5 mm and 15 mm reported as worrisome. , Other predictors of malignant IPMNs include an enhancing solid component/mural nodule(s) and thickened septae or walls.