Inferior Vena Cava
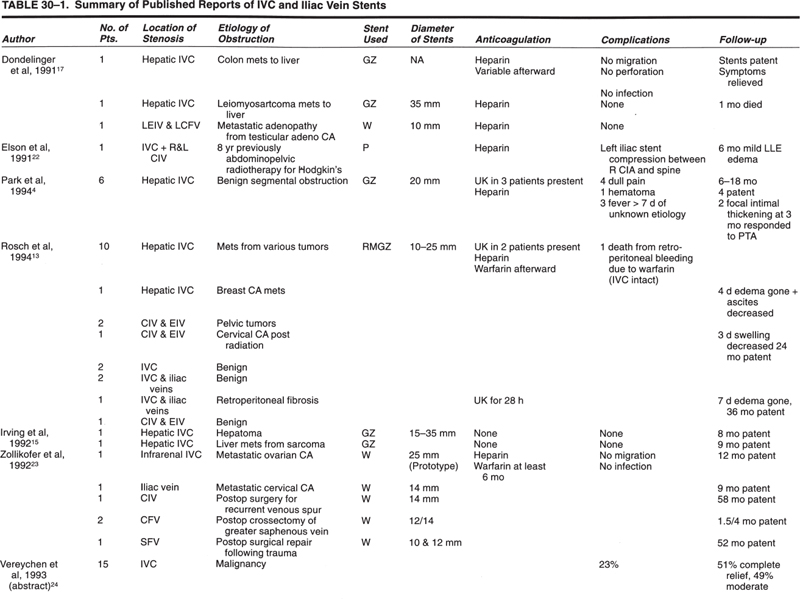
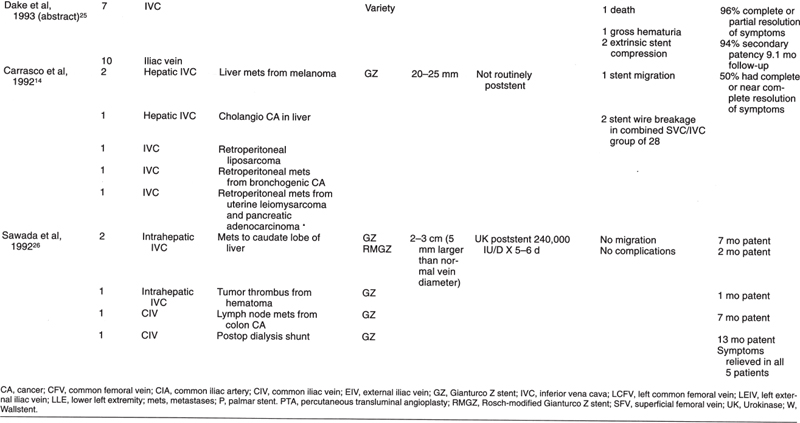
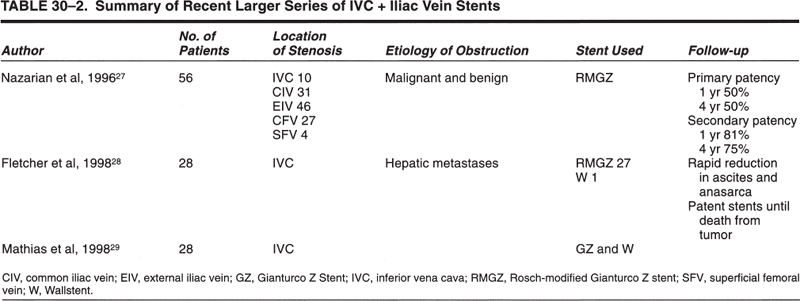
There have been few reports of interventional radiology procedures involving the inferior vena cava (IVC), iliac veins, or leg veins. Many interventional radiology groups may have seen the occasional patient requiring management in these areas. Previously, most reported patients were part of larger reports (Table 30–1) on central venous disease that discussed mostly the superior vena cava (SVC) and upper-extremity veins. Since the first edition of this chapter, some larger series of reports have appeared in the literature concerning IVC, pelvic, and lower-extremity venous severity (Table 30–2).
Just as there is an SVC syndrome of clinical findings associated with SVC obstruction, there is an IVC syndrome associated with infradiaphragmatic venous obstruction. The clinical findings will depend on the level of obstruction (Fig. 30–1), on the severity and length of time of obstruction, and on the presence or absence of collaterals.
When a venous stenosis is located below the diaphragm, how can it be assessed to determine the necessity for treatment? We are accustomed to using the percent of stenosis as a guide in the arterial system, and the same can be used in the venous system. A 70% stenosis is usually hemodynamically significant; a 40% stenosis may not be. In the arterial system, hemodynamic significance can be defined as a systolic gradient of 15 mm Hg or greater or a rise in systolic gradient of 10 mm Hg or greater with vasodilatation.1 Using mean pressures, a 5 mm Hg mean gradient is significant arterially. We have thus become familiar with and have set standards for arterial pressure gradients, but we have much less experience with standards for the venous system. Already a low-pressure system, with much greater variation in anatomy, a large capacitance, a high propensity to rapid collateral formation, and a large respiratory variation, many variables affect the pressure measurements across a venous stenosis. On the venous side, the absolute pressures are lower; therefore, the value for a significant gradient will be lower. (Of course, in patients with arteriovenous (AV) dialysis fistulae and central venous stenoses, the absolute pressures and gradients will be higher). In our clinical experience, we usually consider a mean gradient less than 3 mm Hg to be insignificant in the venous system. A mean gradient of 3 to 6 mm Hg is possibly significant, 6 to 9 mm Hg probably significant, and greater than 9 mm Hg significant. Another way of expressing a significant gradient in the venous system is to say that a gradient of more than 50% of the absolute pressure is significant.
The other factor that makes assessment of the hemodynamic significance of venous stenoses difficult is the large capacitance of the venous system and the presence of collaterals. We know that patients who have venous narrowings may reach a point when they develop swelling and edema peripheral to the stenosis. The swelling can be precipitated by thrombosis or by the reaching of a critical stenosis. In some patients, the symptoms can abate with no treatment at all as a result of the development of collaterals over several days. Other patients have no resolution of symptoms, presumably because of the presence of thrombus or poor collaterals. Patients with persistent symptoms and those in whom patency of the primary venous system is important are those whom we see for management.
Percutaneous transluminal angioplasty (PTA) has limited use in the venous system compared with the arterial system because of the (1) thinner vascular wall and thinner muscular portion of the wall, (2) lower intravascular pressure, and (3) plastic flow in the venous system resulting in marked variations in luminal diameter with respiration.2 Balloon angioplasty in the arterial system is used to manage intimal disease by cracking intimal plaque and stretching the media.3 In contrast, venoplasty is done in an attempt to open the vein against a swollen liver, against a tumor compressing or invading the vein, or against external scarring from previous surgery or radiation. Sometimes we can stretch a vein in these circumstances, but the result will be temporary. Recurrence usually occurs within moments, although an effect may last a few months.
Venous angioplasty may be useful in three specific areas:
1. Stenoses associated with AV dialysis grafts (discussed in another chapter).
2. The most successful infracardiac venous angioplasty seems to be in membranous congenital IVC webs, where success rates of 90% on follow-up as long as 6 years can be seen.4 Stents are used for primary or secondary PTA failures.5 Patients with the type 2 segmental obstruction seem to require stent placement for long-term patency.
3. Another situation in which venous angioplasty may be useful is at anastomoses or postoperative clamp sites where the narrowing is due to some thrombus or intimal hyperplasia. The only way to know is to dilate gently and see what happens to the pressure gradient and to the venogram (Fig. 30–2). Zajko et al6 reported on the safety and efficacy of IVC and portal venous angioplasty in liver transplant patients. The portal vein anastomotic stenoses responded very favorably. The IVC anastomotic narrowings required repeat dilatations or stenting. Pfammatter et al7 recommend PTA for chronic transplant stenoses and stenting for early postoperative stenoses that may be associated with caval torsion or kinking.
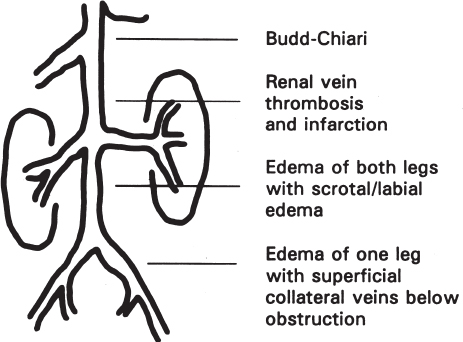
FIGURE 30–1. The inferior vena cava syndrome is associated with infradiaphragmatic venous obstruction. This diagram shows the variation in symptoms, depending on the level of obstruction.
In patients with iliac or IVC thrombosis, thrombolytics can be of help in delineating the underlying etiology of the occlusion (see Table 30–3), which then may be treated. An underlying stenosis caused by tumor, previous surgery, radiation, or a congenital web can be uncovered and treated to avoid rethrombosis, akin to the use of thrombolytics in the arterial system.8 Although there is concern about the potential for pulmonary embolus when lysing a large volume of clot in the IVC, the clot is usually secondary to a more central stenosis, which is not opened until the clot is totally lysed. Park et al4 obtained ventilation/perfusion lung scans after thrombolytics and angioplasty in two patients and after thrombolytics and stenting in three patients. The scans were negative for pulmonary embolus in all five patients.
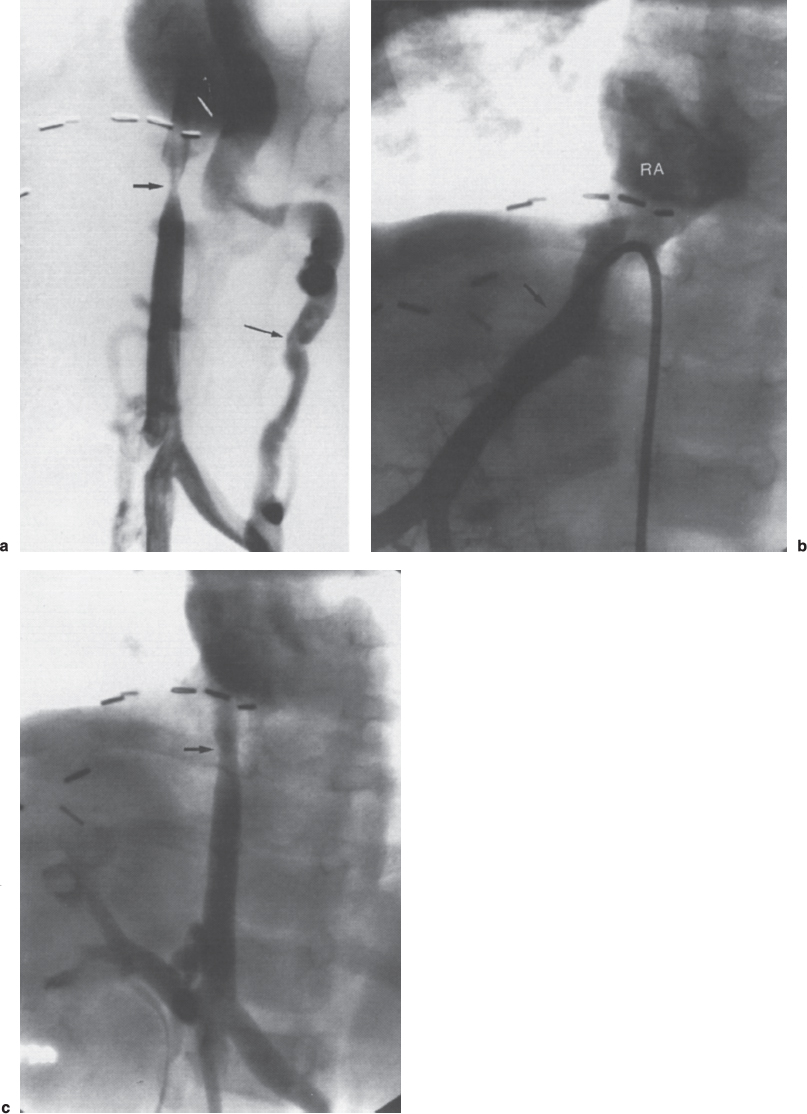
FIGURE 30–2. A 22-month-old girl 15 months after a living-related liver transplant and developed an enlarged liver. Ultrasound suggested inferior vena cava (IVC) occlusion, with patent hepatic veins. An inferior vena cavogram was done to evaluate for a transplant anastomotic web in the IVC above hepatic veins, thereby causing Budd–Chiari syndrome. (a) The inferior vena cavogram did show an IVC stenosis (short arrow) with an 8 mm Hg gradient and markedly enlarged collaterals (long arrow). (b) The hepatic venogram showed patent hepatic veins (arrows) with free flow into the right atrium (RA) above the level of the IVC stenosis. Biopsies were taken of the liver to evaluate the etiology of hepatomegaly. The IVC stenosis was dilated to improve vena caval and renal vein flow. It was opened easily with a 4-mm-diameter balloon. (c) A post-percutaneous transluminal angioplasty (PTA) inferior vena cavogram shows an improved lumen (arrow) with no collaterals and final gradient of 3 mm Hg.
Dake9 reported on catheter-directed thrombolysis of the lower extremities from the jugular approach. Thrombolysis was followed by angioplasty and stent placement to treat the underlying vein stenosis.
When assessing a thrombosed iliac vein or IVC for thrombolytic therapy, it is important to assess both the inferior and superior extent of the clot, which usually requires catheterization from both femoral and upper extremity or internal jugular approach. For IVC or iliac vein clot, we usually place our catheter from the femoral approach and embed the catheter into the clot. As of this published date, we no longer have urokinase available, and now use tissue plasminogen activator (TPA). We usually will “lace” the clot with 5 mg (5 cc) or 10 mg (10 cc) of TPA, followed by an optional high dose (.04 mg/kg/hr) for 4 hours, followed by a lower-dose (.02 mg/kg/hr) infusion. The recommended dose of concomitant heparin is currently in flux. We would use from 500 μ/hr to full heparinization, depending on the patient risk.
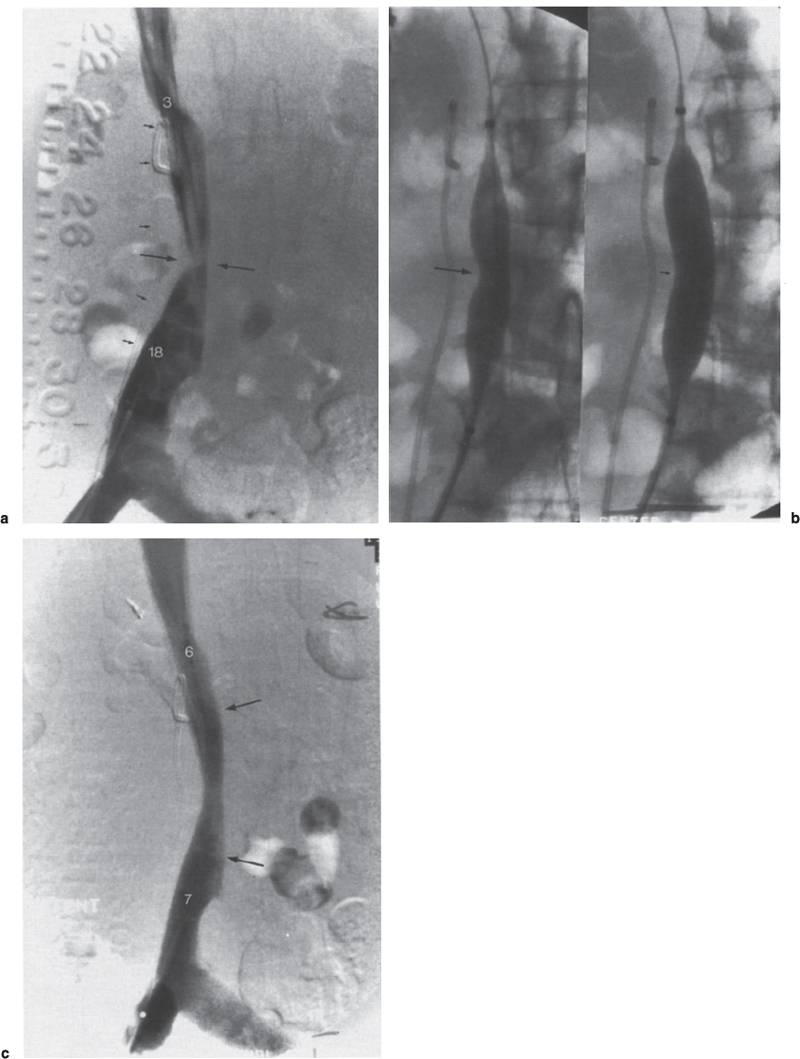
FIGURE 30–3. (a) A 58-year-old woman with recurrent pancreatic cancer and a gradual increase in bilateral lower extremity swelling. Inferior vena cavogram shows focal eccentric stenosis at L3 (long arrows). Note the double J right ureteral stent (small arrows) placed previously for ureteral stricture at the same level. Pressure gradient across the inferior vena cava (IVC) stenosis was 15 mm Hg. Numbers represent actual pressures in mm Hg. (b) Prestent 12-mm balloon dilatation of IVC demonstrates balloon “waisting”(long arrow) with small residual eccentric narrowing at full inflation (small arrow). Post-percutaneous transluminal angiography (PTA) gradient was still 14 mm Hg. (c) Inferior vena cavogram after placement of 16 mm × 4 cm Wallstent (between arrows). Note the reduction in gradient from 15 mm Hg before stenting to 1 mm Hg post-stenting.
Surgical reconstruction of the large veins is difficult compared with the arterial system. Prosthetic grafts are susceptible to failure because of the low pressure and low velocity of flow in the venous system.10
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree