There are several vascular ultrasound technologies that are useful in challenging diagnostic situations. New vascular ultrasound applications include directional power Doppler ultrasound, contrast-enhanced ultrasound, B-flow imaging, microvascular imaging, 3-dimensional vascular ultrasound, intravascular ultrasound, photoacoustic imaging, and vascular elastography. All these techniques are complementary to Doppler ultrasound and provide greater ability to visualize small vessels, have higher sensitivity to detect slow flow, and better assess vascular wall and lumen while overcoming limitations color Doppler. The ultimate goal of these technologies is to make ultrasound competitive with computed tomography and magnetic resonance imaging for vascular imaging.
Key points
- •
Innovative vascular ultrasound technologies offer improved visualization of small vessels and higher sensitivity to slow flow.
- •
Just as in Doppler ultrasound, image optimization and recognition of artifacts are key to accurate interpretation.
- •
New vascular ultrasound techniques continue to develop and will have more applications with additional research and experience.
- •
Contrast-enhanced ultrasound requires an intravenous line for administration.
Introduction
Ultrasound (US) is a powerful tool for evaluating vasculature because it provides valuable information regarding blood flow and vessel morphology. The benefits of US are well recognized, including portability, availability, cost-effectiveness, and lack of ionizing radiation. Challenges common to all US technologies are limited penetration in bariatric patients, poor acoustic windows, and user dependence. Because of its importance in vascular imaging, there have been many recent innovative advances in US techniques meant to improve the diagnostic capability of US.
Doppler US—including its 3 main modes: color Doppler, spectral Doppler, and conventional power Doppler—has been a part of most US examinations for more than 40 years and its advantages and challenges are well documented. Color Doppler US (CDUS) provides information about flow, including direction of flow within a selected box superimposed on the gray-scale US image and mean blood flow velocity. Spectral Doppler US has the added benefit of providing the absolute velocity of blood flow within a small sample gate placed in a vessel. The spectral tracing gives vital information on hemodynamic values of blood flow, such as acceleration time and peak systolic and diastolic velocities, which can be used to calculate resistive indices. Conventional power Doppler US (PDUS) is more sensitive to slow flow than color Doppler and provides detail on the strength of the Doppler signal within an area but lacks directional information. PDUS is most useful in assessing global perfusion of an organ. Although all these modes are useful in assessing and diagnosing vascular pathologies, some of their capabilities are limited, especially in the assessment of vascular beds that demonstrate very slow flow and pathologies that are associated with vascular wall and lumen.
Challenging vascular systems that are difficult to evaluate using conventional Doppler modes or equivocal Doppler cases require more advanced US applications with the highest sensitivity to flow to provide more definitive and accurate diagnosis. Newly developed innovative US techniques emphasize the role of US in accurate diagnosis of various vascular pathologies and conditions, including assessment of atherosclerotic plaque and visualization of plaque ulceration, detection of significant endoleaks in patients post–endovascular aneurysm repair (EVAR), identification of patency of arterial and venous flow in organ transplants, and accurate diagnosis of near occlusion and its differentiation from complete occlusion of either central or peripheral arterial or venous bed. In many of aforementioned pathologic conditions, a reliable US tool is imperative in improvement of diagnostic capability of US and reliability of the imaging findings. Additionally, a safe and highly diagnostic technique is desired for screening of vascular pathologies and for offering a sensitive and robust tool for a lifetime follow-up of major vascular conditions.
Most of the advanced US vascular applications were designed with the overall goal of troubleshooting absence of flow detection in order to avoid false-positive diagnoses of vascular occlusion and increase confidence of an interpreter to an accurate identification of vascular pathology. This article is a review of the latest innovations in vascular US with examples of their applications as well as a discussion of their strengths and weaknesses ( Table 1 ).
| Application | Pros | Cons |
|---|---|---|
| Color Doppler | Provides information on presence or absence of flow, direction of flow, mean flow velocity, gray-scale tissue characteristics | Absolute velocity cannot be determined. Motion outside of a vessel may be seen as flow. Color can bloom beyond the wall of the vessel. Angle dependent |
| Spectral Doppler | Provides information on velocity and direction of flow Provides quantitative assessment of flow (acceleration, peak systolic velocity, end diastolic velocity, etc.) | Only a small area can be assessed. Angle dependent |
| Conventional power Doppler | More sensitive to slow flow than color Doppler Enables global assessment of perfusion Not angle dependant | Does not provide direction of flow or velocity Sensitive to motion artifact of surrounding tissues |
| CEUS | No nephrotoxicity Excellent safety profile Can repeat dose Provides information on flow in microvasculature Aids in differentiating benign and malignant thrombus | Minimally invasive (intravenous) Gray-scale imaging is degraded by low mechanical index. More time consuming |
| B-flow | Based on gray-scale US and amplifies signal of moving blood Can subtract gray-scale background Provides information of all vessels in field of view, including small and slow-flow vessels regardless of angle | Does not provide direction of flow or velocity. |
| MVI | Based on PDUS Provides information on very small vessels by reducing background clutter and maintaining high frame rates | Detection of flow can be affected by adjacent high velocity and pulsating vessels. Some of the applications do not provide absolute velocity calculation. |
| PAI | Newly emergent molecular imaging, based on combination of thermoelastic expansion of light energy (laser) and sonographic beam production. Provides information on blood oxygen saturation and hemoglobin, capable of measuring blood velocity and detection and distribution of biomarkers; visualizes blood vessel structure and associated plaque | High resolution is at the expanse of depth of penetration. A majority of applications still are preclinical. |
| IVUS imaging | Provides intraluminal assessment of a vessel lumen, wall and anatomy; plaque distribution and size; allows measurement of the intravascular diameter; offers guidance for intravascular procedures, including stents and graft placement, assists in fenestration of dissection flap | No information about the outer vascular wall and adjacent structures |
| Elastography, strain and shear wave | Provides assessment of arterial wall elasticity Offers assessment of chronicity of blood clot | Reproducibility still varies No defined threshold values or criteria available at this time |
| 3-D vascular imaging | Offers assessment of atherosclerotic plaque volume and intrinsic characteristics: composition, surface irregularity, size, and dimensions Improved ability to estimate flow volume and more accurate assessment of degree of stenosis | Errors in calculation of flow volume due to vascular motion and pulsation |
Image optimization and AutoScan function
With the new era of US vascular imaging, most US units are now equipped with automatic image optimization (AutoScan) capabilities. One of the most common advantages of the AutoScan function is automatic localization and identification of a vessel within the area of scanning on color Doppler and placement of the spectral gate in the center of the identified vessel. Adjustment of gain, velocity scale, wall filter, and color box also is automatic. Although this automated function is less operator dependent, in challenging cases manual override may be necessary for optimal image acquisition and correct interpretation. Therefore, knowledge of and expertise in Doppler optimization parameters remain significant and necessary to ensure the performance of high quality Doppler examinations. , In addition, just as with the traditional Doppler, image optimization is imperative when new advanced techniques and applications are used. Thus, for microvascular flow, contrast-enhanced US (CEUS), and B-flow imaging (BFI), selection of appropriate parameters, such as acoustic window, gain, zone, focus, depth, transducer choice, and in some cases angle of insonation, is critical for optimal scanning. Moreover, recognition of modern vascular application specific artifacts and understanding of their significance as well as how to avoid them also are paramount in accurate assessment of flow.
Directional power Doppler
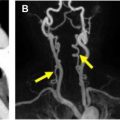
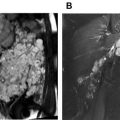
Directional power Doppler is a relatively new application that is based on the conventional power Doppler mode. In addition to providing information regarding presence of slow flow and global perfusion of an organ or vascular structure, it also allows determination of flow direction ( Fig. 1 ). This is particularly important in the field of obstetric US, where accurate assessment of vessel patency as well as flow pattern and direction have a significant impact on patient and fetal management. For example, assessment of flow direction in the umbilical cord is of critical importance in the setting of multigestation pregnancies and in the evaluation of twin-twin transfusion. Additionally, directional power Doppler plays a significant role in the assessment of neovascularity within visceral organ masses as well as when there is concern for a postintervention arteriovenous fistula in a native or transplanted visceral organ. , Directional power Doppler increases the diagnostic confidence of radiologists in the assessment of complete occlusion of key vasculature, such as the carotid and portal vein systems, also allowing for accurate differentiation of occlusion from near occlusion or stasis (as in cases of a carotid string sign or slow flow in the portal venous system). Directional PDUS, however, has limitations similar to those of conventional PDUS. For example, it does not provide an estimation of absolute or mean velocity, it is susceptible to significant motion artifact, and it is affected by the Doppler angle. A significant disadvantage is that, as in conventional power Doppler, directional PDUS cannot produce aliasing, making it difficult to quickly identify areas of potential vascular high-level stenosis. One of the differentiating features between conventional and directional power Doppler ultrasound is that conventional PDUS is independent of the angle of insonation. Utilization of a combination of new applications is becoming a trend in vascular imaging. For example, there is an interest in the use of directional power Doppler in conjunction with 3-dimensional (3-D) rendering techniques and contrast agents, which show great promise for the evaluation of vascular beds.

Contrast-enhanced ultrasound
Although CEUS has been readily available in Asia and Europe, it has been slow to gain acceptance in the United States in part due to regulatory obstacles and in part because of heavy reliance on computed tomography (CT) and magnetic resonance imaging (MRI). The Food and Drug Administration only approved the use of Lumason (Bracco Diagnostics, Monroe Township, New Jersey) for characterization of liver lesions in 2016.
CEUS agents consist of gas-filled microbubbles surrounded by a supportive shell usually made of phospholipids. These agents are metabolized exclusively by the liver and lungs and are not nephrotoxic, making them safe for use in patients with renal insufficiency and allergies to other contrast agents. No laboratory tests are required before use. Microbubble US contrast agents have an excellent safety profile with a severe adverse reaction rate of 0.007% to 0.009%, which is comparable to that of gadolinium and superior to that of iodinated contrast agents. ,
Challenges specific to CEUS include special software necessary on the machine to perform contrast-enhanced examinations. This is available on all major US vendors. An intravenous line must be placed for administration of the contrast agent and there must be an additional person available to inject the contrast. There is a learning curve to optimize CEUS imaging, which includes timing of the vascular phases after injection for imaging of masses, properly decreasing the gain before administering contrast to avoid artifactual enhancement of background tissue, and minimizing mechanical energy imparted into the area being imaged. Microbubbles are sensitive to mechanical energy and burst with increased mechanical index or continuous imaging in one place.
CEUS agents are smaller than a red blood cell and remain purely intravascular. , CEUS imaging provides dynamic, real-time imaging of vasculature along with excellent spatial and temporal resolution. Because of the excellent safety profile of these contrast agents, the dose can be repeated in the same session. These characteristics makes CEUS great for vascular and microvascular imaging. Some of the vascular applications of CEUS are evaluating endoleaks in patients post-EVAR, assessment of vascular thrombus, and vessel patency.
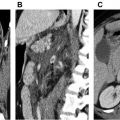
Surveillance after Endovascular Aneurysm Repair
Approximately 70% of abdominal aortic aneurysm repairs are performed using EVAR procedures as opposed to the more invasive open technique. EVARs require perpetual surveillance for graft-related complications, which occur in approximately 40% of patients within 4 years of EVAR. CT angiography is considered the gold standard for surveillance, but it is expensive and uses ionizing radiation and iodinated contrast. CDUS can detect a change in the aneurysm sac diameter but is less sensitive for detection of endoleak compared with CT angiography. A meta-analysis demonstrated a 0.72 sensitivity and 0.95 specificity of CDUS to detect an endoleak. CEUS showed a higher sensitivity of 0.91 and a slightly lower specificity of 0.89, meaning that CEUS was better to ruling in an endoleak ( Fig. 2 ). The drawback of these techniques were that a majority of endoleaks detected were type II, which often do not require intervention as opposed to type I or III endoleaks. The addition of 3-D to CEUS evaluation of the EVAR repair shows promise as being equivalent to CT angiography in detecting and classifying the type of endoleak. Taken together, CEUS may be an appropriate examination for follow-up of an EVAR procedure with CTA reserved for further evaluation of suspicious findings.

Challenges with this examination can include finding an appropriate acoustic window that would allow appropriate visibility of the EVAR stent graft and ultrasound beam penetration. The examination should be performed in a fasting state to limit interference by bowel gas. CEUS examination can also be challenging in bariatric patients.
Evaluation of Portal Vein Thrombus
Hepatocellular carcinoma (HCC) is the third most common cause of cancer death in the world. Bland portal vein thrombus can be seen in 5% to 26% of cirrhotic livers and in up to 42% of patients with HCC. Tumor in vein (also known as tumor thrombus) occurs in 10% to 60% of patients with HCC. Distinguishing between bland and tumor thrombus is diagnostically important because it influences prognosis and is a contraindication to liver transplantation. Tumor thrombus can be difficult to detect by gray-scale US in the background of a heterogenous, cirrhotic liver. Slow flow in the portal vein can mimic thrombus on CDUS and, although detection of an arterial spectral Doppler tracing is 100% specific for tumor thrombus, the sensitivity is approximately only 20%. In 1 study of 54 patients with known HCC and portal vein thrombus, the addition of CEUS to detect tumor in vein increased sensitivity to 88% compared with CDUS, with a sensitivity of 20%. The real-time, dynamic evaluation of the portal vein with CEUS and the ability to repeat the contrast dose during examination makes this a valuable adjunct to US, which often is a part of the HCC screening program ( Fig. 3 ).

Transplant Artery Patency
Given the small size of the hepatic artery and it main branches, accurate diagnosis of occlusion of the transplant hepatic artery may be challenging. Hepatic artery thrombosis (HAT) is the most common and one of the most devastating vascular complications in liver transplant and must be recognized early for appropriate treatment and management in order to save the graft. Part of the difficulty in diagnosing hepatic artery occlusion using conventional US is due to its close proximity to the portal vein. A known limitation of CDUS is the blooming of color beyond vascular walls, which in this case means that the flow in the larger portal vein can obscure the hepatic artery. Based on the published literature, , , , there is clear evidence that CEUS improves the diagnostic assessment of HAT and hepatic artery stenosis, compared with conventional US. CEUS also has been shown to be a more optimal choice when comparing it to other imaging modalities. Thus, 1 study showed an increased sensitivity of CEUS to detect HAT compared with CT angiography, with sensitivities of 89% and 82%, respectively.
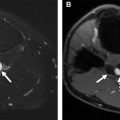
Renal transplants also are well suited for evaluation by CEUS, especially because the use of iodinated contrast often is avoided to protect renal function. Vascular complications of renal transplants range from renal vein thrombosis, which happens early after transplant, to renal artery thrombosis, which is a very rare occurrence, to renal artery stenosis, which is a later but not uncommon complication occurring in approximately 3% of renal transplants. Renal transplants commonly are followed with CDUS for evaluation of potential vascular complications but the gold standard remains digital subtraction angiography, which is an invasive technique. CEUS can provide important information about the patency of the transplant vasculature as well as the vessel caliber and assess for the presence of arterial or venous stenosis. CEUS also can be useful in detecting areas of infarct ( Fig. 4 ).