Fig. 1
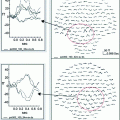
The premovement subperiods and the onset of the rectified EMG. The preparatory period was divided into five subperiods from the onset of EMG to 4000 ms before movement. The time scale is expressed in minus values before the onset of movement. Stimulation of the median nerve was applied at random and the MEG signals following stimulation were averaged separately depending on each subperiod to obtain the premovement somatosensory evoked magnetic fields (SEFs). The sources were located in the posterior bank of the central sulcus in the hemisphere contralateral to the side stimulated. The graphs show the mean and standard deviation of the ECD moments of the M20 and M35 components in the rest condition and premovement subperiods. ** p < 0.01; Statistical significance compared with the values in the rest condition, # p < 0.05, ## p < 0.01; Statistical significance compared with the values for the 4,000–3,000 ms subperiod before EMG onset. Two periods for the M35 showed a significant reduction as compared with the rest condition and/or the 4,000–3,000 ms subperiod before EMG onset. The M20 showed no significant change. Adapted from Wasaka et al. (2003)
Interestingly, the time course of the M35 modulation, starting from 1500 ms before the movement and the remarkable attenuation just prior to the movement, was similar to that of the activities of movement-related cortical potentials that reflected the neural activities of movement preparation in motor-related areas. In addition, our previous study showed that the extent of the centrifugal mechanism for SEPs was dependent on the amplitude of the negative slope. This result suggested that the centrifugal modulation in the SI was related to the activities of motor related areas (Wasaka et al. 2005b). Subdural recording showed that the supplementary motor area (SMA) and the MI activities in this period were recorded from the cortex of humans (Ikeda et al. 1992). Motor related areas, such as SMA and MI, have extensive cortico-cortical connections to other cortices such as the SI (Jones et al. 1978) and possibly other sensory associated cortices. Intracortical microstimulation of the neurons in the MI in monkeys caused a profound decrease in the magnitude of the short-latency component of somatosensory evoked potentials (Jiang et al. 1990), suggesting that the activities in the motor related areas just before movement could modulate the response in the SI, especially the generator for the M35 component. It is assumed that these electrophysiological changes are associated with a decrease in tactile sensitivity commonly observed before the onset of movement of the limb that received the sensory stimulation (Schmidt et al. 1990).
5 Modulation in the SII During the Preparatory Period of Voluntary Movement
There is no consensus as to the function of the SII concerning sensorimotor integration during voluntary movement. Enhancement of SII activation was observed (Huttunen et al. 1996; Forss and Jousmaki 1998; Lin et al. 2000) and it was assumed that this phenomenon reflects tuning of the SII neurons to relevant somatosensory information from the regions of contracting muscle. On the other hand, some researchers reported an attenuation of SII activation (Avikainen et al. 2002; Inoue et al. 2002). Using a self-initiated movement task to investigate the preparatory period, whereby the centripetal effect on the SEF response can be eliminated, one can mainly examine the centrifugal effect. We showed an enhancement of SII activation in the 0 to –500 ms sub-period (Fig. 2) (Wasaka et al. 2005a).
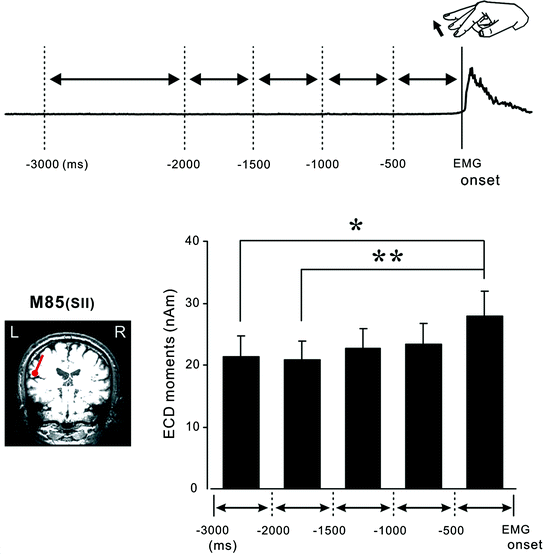

Fig. 2
The premovement subperiods and the onset of the rectified EMG. The preparatory period was divided into five subperiods from the onset of EMG to 3000 ms before movement. The time scale is expressed in minus values before the onset of movement. The dipole for the 80 ms response was identified in the temporal region, corresponding to the SII cortices. The graph shows the mean and standard error of the dipole moment of the SII contralateral to electrical stimulation in the premovement subperiods. * p < 0.05, ** p < 0.01; Statistical significance within two pairs. The dipole moment for the SII was significantly larger in the 0–500 ms subperiod than the 1500–2000 ms or 2000–3000 ms subperiod before EMG onset. Adapted from Wasaka et al. (2005a)
It is generally agreed that attention to somatosensory information enhances activation of the SII cortex (Hari et al. 1990; Mauguiere et al. 1997; Fujiwara et al. 2002). Although it is hard to eliminate the attentional effect, we instructed the subjects to concentrate on self-initiated finger extension and not to pay attention to electrical stimulation. Subjects reported that they concentrated on the finger extension and did not turn their mind to the electrical stimulation throughout the recording session. Therefore, although it is possible that attention contributed in a small way to the enhancement of SII activation, the activation of motor related areas prior to voluntary movement enhances the cortical effects of the SII either by increasing synchronicity or by increasing the number of neurons active via the centrifugal process.
6 Differential Modulation in the SI and SII Preceding Voluntary Movement
In the period of 500 ms before the onset of self-initiated movement, an attenuation of activation in the SI and enhancement in the SII was found. The opposite effects of movement on SI and SII cortices indicated that the motor and higher-order brain systems regulate sensory information at several processing stages by the centrifugal process. Motor commands can facilitate or suppress sensory responsiveness and, thus, probably perception, depending on temporal and behavioral constraints.
Removal of the SI area seriously impaired the processing of tactile information in the SII in macaques (Pons et al. 1987). However, deactivation of the SI did not have clear effects on the responsiveness of the SII (Zhang et al. 1996). In addition, tactile information could be directly conveyed to both SI and SII cortices from overlapping regions within the ventral posterior nucleus of the thalamus (Zhang et al. 2001). In humans, MEG responses from SII increased with active attention, while little effect of attention was observed in the SI (Mima et al. 1998; Fujiwara et al. 2002). Moreover, the responses in the SI and SII are modulated differently depending on the intensity of the electrical stimulation (Torquati et al. 2002; Lin et al. 2003). From these results, it appears that somatosensory information processing, concerning sensorimotor integration in SII, may be independent of that in SI.
Our sensory systems are constantly bombarded by numerous sensory stimuli, from which we must extract the few stimuli important to the control of our movement. One can therefore recognize that an attenuation of SI activation is involved in filtering information. Although much attention has been given to sensorimotor integration in the SI, there is little evidence of such a phenomenon in the SII and the role of the SII in motor execution has not been fully elucidated in humans. Compared with the SI, SII is speculated to serve a higher level of cognitive function in somatosensory information processing, such as attention, decision-making, object recognition, and the integration of nociceptive and non-nociceptive inputs (Mima et al. 1998; Steinmetz et al. 2000; Romo et al. 2002; Inui et al. 2003; Qiu et al. 2004). Our results showed that these cortical areas play a different functional role in sensorimotor integration. When we are moving, the sensory threshold is attenuated. By contrast, exploration using fingertips is sensitive during movement execution (active touch). This neural mechanism can be explained by an enhancement of SII activation. To clarify the function of SII concerning sensorimotor integration, we conducted further research.
7 Crossmodal Interaction Between Somatosensory and Visual Information
Crossmodal interaction occurs when neural activity from one sensory modality modulates activity in another (Macaluso et al. 2000; Kida et al. 2007). Crossmodal links between visual and somatosensory information have shown the critical role of vision in determining limb position and localizing tactile sensations (vanBeers et al. 1996; Botvinick and Cohen 1998; Graziano 1999). For example, viewing a body part improves tactile perception and facilitates the amplitude of long-latency components of event-related potentials (Taylor-Clarke et al. 2002; Cardini et al. 2011). In addition, there is evidence that vision of the body is crucial for localization of tactile stimuli (Eimer et al. 2004; Sambo et al. 2009).
Although less attention has been devoted to the effect of observation of movement on information processing in somatosensory areas, some studies have reported neural modulation in SI and SII. Previous studies showed that viewing another person’s gestures modulates the excitability of somatosensory areas (Avikainen et al. 2002; Rossi et al. 2002; Mottonen et al. 2005; Pihko et al. 2010). These results indicate that the somatosensory areas are involved in the mirroring of actions.
8 Somatosensory and Visual Interaction During the Execution of Voluntary Movement
Recognizing one’s own movement is essential to the control of voluntary movement. Movement causes changes to sensory inflow as well as changes in the position of body parts. The movement of one’s body parts is perceived not only by visual information but also by somatosensory feedback from muscles, skin and tendons which provide information on the status of each part being moved in a moment. Under normal conditions, the visual estimate of limb position is congruent with the somatosensory estimate and motor command, and movement is usually achieved automatically without awareness of the component processes. By contrast, in novel motor tasks or situations which produce conflict or incongruence between intentions and sensorimotor consequences, the mismatch between the actual sensory feedback and predicted movement of the body part disrupts motor execution. A crucial issue is elucidating the brain mechanisms that integrate the multi-sensory information and motor commands for motor control.
It has been suggested that a copy of the motor signal, known as an efference copy, is created so that sensory signals generated from external stimuli can be distinguished from reafferent signals from body movement (von Holst and Mittelstaedt 1950; Wolpert et al. 1998). Corollary discharges are produced only if the motor commands interact with unpredicted sensory inputs and inhibit the neural response to self-generated sensory signals (Sperry 1950). More activity in somatosensory areas was found when an unpredicted stimulus was externally delivered (Hesse et al. 2010). Since crossmodal interaction between somatosensory and visual inputs exists in the somatosensory areas, there is considerable validity to the notion that the prediction of visual feedback of movement modulates the somatosensory areas.
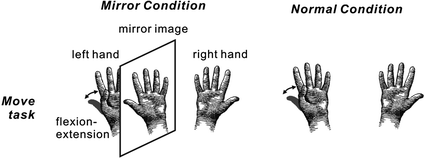
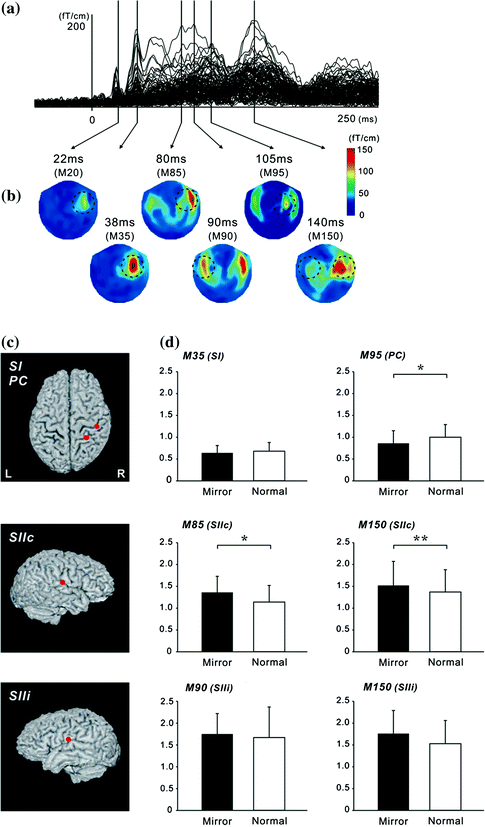
We investigated whether activation in somatosensory areas was affected by discordance between an intended and executed action. A mirror box creates unintended visual feedback of body movement (Fig. 3). Subjects inserted their hands into the mirror box with the forearm supine (Mirror condition). The position of the right hand was adjusted so that the mirror image precisely overlapped the view of the masked left hand. Since the actual visual information on the left hand was masked by the mirror, a mirror image of the right hand was provided. In the Normal and Mirror conditions, subjects experienced the appropriate somatosensory feedback, but in the Mirror condition, what they watched was incongruent with the expected visual feedback which produced a state of cognitive conflict. The motor task was a self-paced thumb movement of the left hand. Electrical stimulation for the recording of somatosensory responses was delivered to the median nerve at the left wrist. Subjects watched the stationary image of the hand while they performed the self-paced movement of the left thumb. In this situation, subjects felt that the movement was not controlled by themselves or the moving body part did not belong to them. The cortical response showed that neural activation in the SII and parietal cortex was strongly affected by the unexpected visual feedback (Fig. 4) (Wasaka and Kakigi 2012a; Wasaka and Kakigi 2012b). The SII showed significantly higher activation with unpredicted visual feedback of movement, whereas the opposite was true of the parietal activation. These results provide evidence that the visual information modulates activation in somatosensory areas during voluntary movement.
Get Clinical Tree app for offline access

Fig. 3
Schema of the experimental paradigm. In the Mirror condition, subjects inserted their hands into a mirror box with the forearm supine. The position of the right hand was adjusted so that the mirror image precisely overlapped the view of the masked left hand. A mirror image of the right hand was presented instead of the left hand. Subjects performed self-paced continuous and repetitive flexion-extension of the left thumb in the normal visual feedback (Normal condition) and the incongruent non-veridical visual feedback condition (Mirror condition). Electrical stimulation for the recording of somatosensory responses was delivered to the median nerve at the left wrist. Adapted from Wasaka et al. (2012a)

Fig. 4
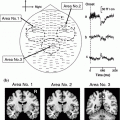
Superimposed MEG waveforms and topographical maps. a Superimposed root sum square (RSS) waveforms from 102 sensors. b Map of the topography of the RSS at the peak components. The first cortical activation was identified around the central area contralateral to the hemisphere of the side stimulus (M20 and M35). Then, bilateral activations were identified in temporal areas at around 80–100 ms (SII). PC activity was identified in the centro-parietal area located posterior to the SI activity. c The location of equivalent current dipoles in each component superimposed on 3D images. d




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree