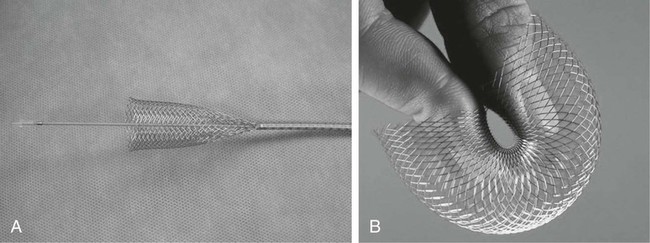
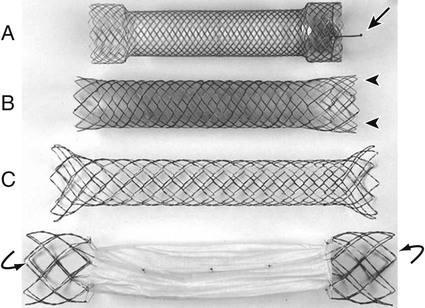
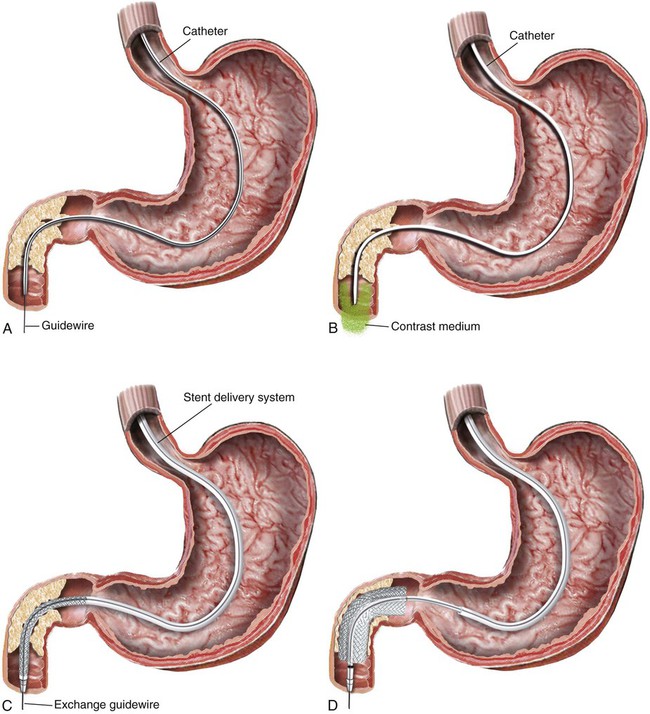
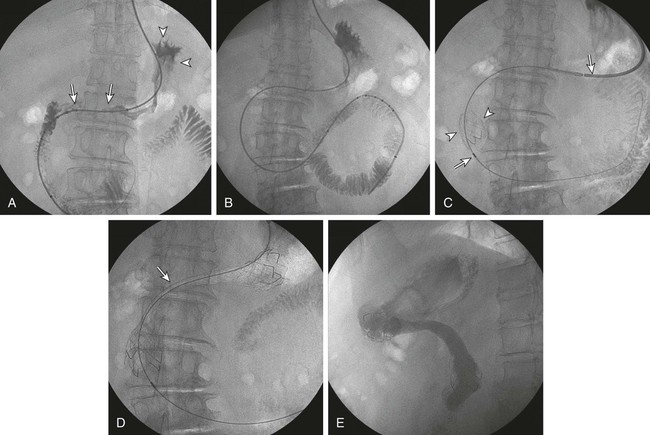
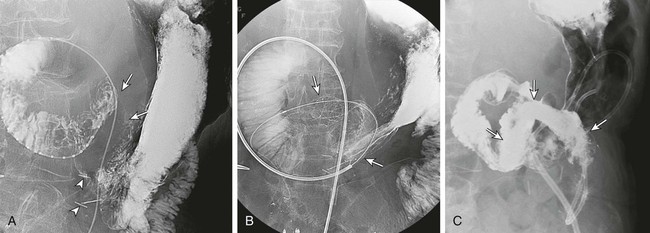
Gastric outlet obstruction is a preterminal complication of advanced malignancies of the pancreas, stomach, and duodenum. Patients with gastric outlet obstruction experience intractable nausea, vomiting, and anorexia, which may in turn cause electrolyte imbalance, dehydration, and malnutrition. Furthermore, these patients are at constant risk for aspiration and pneumonia. The primary goal of treatment is palliation of obstructive symptoms, thereby improving quality of life.1 Although surgical gastrojejunostomy with or without gastrectomy has been the traditional method of palliative treatment, many patients are unfit for bypass surgery because of poor medical condition at initial evaluation. Moreover, it carries a significant rate of morbidity and mortality and is associated with persistent or delayed relief of symptoms and prolonged hospital stay.2 Nonsurgical palliation by means of drainage via a nasogastric tube or gastrostomy does little to improve a patient’s quality of life. Placement of self-expandable metallic stents is an established treatment option in patients with malignant biliary and esophageal obstruction. Recently, stents have been increasingly used to treat malignant gastroduodenal obstruction, with successful palliation achieved in most patients. Advantages of gastroduodenal stent placement over surgical palliation include more rapid gastric emptying, fewer complications, and improved quality of life. Anastomotic malignant obstruction after gastrectomy or esophagectomy is also an indication for stent placement.3,4 Although stent placement for benign obstruction is still under investigation, it may be a possible alternative treatment when coexisting morbidity involving the cardiopulmonary system limits surgical treatment.5 The only absolute contraindication to stent placement is gastrointestinal (GI) perforation with peritonitis or tension pneumoperitoneum. Multifocal small bowel obstruction has been reported as the main cause of clinical failure after stent placement.6,7 Therefore, clear evidence of distal small bowel obstruction is also a contraindication to stent placement. Patients with peritoneal carcinomatosis are at high risk for multifocal small bowel obstruction. Both covered and bare stents have been used in the stomach and duodenum. Covered stents have the advantage of resisting tumor ingrowth through the stent mesh wire but are prone to migration. They are rigid and require a large delivery system and thus are difficult to deploy at distant locations along a tortuous delivery route. Advantages of bare stents over covered ones include greater flexibility, requirement for a smaller delivery system, and more resistance to migration. When used for long-term palliation of malignant obstruction, they are more prone to obstruction by tumor ingrowth. However, in the majority of cases, tumor ingrowth has not been clinically important because of the limited life expectancy of the patient. Therefore, to date, bare stents have been used much more frequently than covered stents in patients with gastroduodenal obstruction.7–11 Until recently, because stents dedicated to GI use were unavailable, interventionalists used stents designed for esophageal or vascular use, including the Wallstent (Boston Scientific, Natick, Mass.), Ultraflex stent (Microinvasive/Boston Scientific), Gianturco Z-stent (Wilson-Cook, Winston-Salem, N.C.), and EsophaCoil (Intra-Therapeutics, Eden Prairie, Minn.).7–11 The Wallstent endoprosthesis is the most frequently used stent. Vascular Wallstents are very flexible but have a small diameter (16 mm) inadequate for GI use, whereas esophageal Wallstents are sufficiently large (20-25 mm) but relatively rigid. Recently a Wallstent dedicated to GI use has been developed (Fig. 130-1). Available stents range from 18 to 22 mm in diameter and are mounted on 10F delivery systems 230 cm long for endoscopic placement and 160 cm long for peroral fluoroscopic placement. The enteral Wallstent is a bare stent braided from multiple strands of stainless steel wire. Advantages of this stent include high longitudinal flexibility, adequate radial force, and the fact that it uses a small introducer system. Disadvantages of the enteral Wallstent are substantial shortening (≈40%) after deployment, unavailability in a covered version, and potentially traumatic ends of the stent. More recently, a new enteral stent (WallFlex [Boston Scientific]) was introduced that is made of nitinol instead of stainless steel.12,13 This new stent has been constructed to provide improved flexibility while maintaining lumen integrity, has looped ends to reduce risk of mucosal injury, and has a proximal flared end to minimize risk of stent migration.12,13 The Ultraflex stent is knitted from nitinol mesh wire, which slowly expands when deployed at body temperature and is available in both covered and bare versions.14 It has significantly lower radial expansile force than the other stents, but it is highly flexible and thus can be placed across acutely angled stenoses. The stent is available in a 23-mm middle diameter, has a 28-mm funnel-shaped proximal end, and is mounted on a 16F delivery catheter. The Gianturco Z-stent is available in bare and polyethylene-covered versions. The bare stents are cylindrical or flared at both ends and available in diameters of 15 to 35 mm. The covered esophageal Z-stent is designed with flared ends to prevent migration. The diameter of the stent is 18 mm in its midportion and 25 mm at each end.15 The stent is delivered through a 28F sheath. The greater inflexibility of these stents limits their use in the GI tract. In some recent publications from Korea, use of various types of covered enteral stents (Fig. 130-2) has been reported. The Niti-S stent (Taewoong Medical, Ilsan, Korea) and the HANAROSTENT (M.I.Tech, Pyungtaik, Korea) are woven from a single thread of nitinol wire. The stents are covered with polyurethane (Niti-S) or silicon membrane (HANAROSTENT). The diameters of the body of the stents are 16 or 18 mm, and both ends are flared, which increases the size to 20 to 24 mm. They are mounted on an 18F delivery system.16 These stents are highly flexible and exert adequate expansile force. Recently, Song et al.17,18 developed a nylon-covered stent (Dual duodenal stent [S&G Biotech, Sungnam, Korea]) that was designed to be placed coaxially. It is mounted on a very low-profile (3.8 mm) delivery system. Other authors have introduced a new duodenal bare metallic stent (BONASTENT M-Duodenal [Standard Sci-Tech Inc., Seoul, Korea]), through which biliary stent placement is safe and effective for palliative treatment of malignant biliary and duodenal obstruction.19 For peroral placement, stents can be placed under fluoroscopic or combined endoscopic-fluoroscopic guidance. Advantages of endoscopic stent placement over fluoroscopically guided placement include direct visualization of the lesion and greater accessibility to the obstruction by straightening of the access route through the distended stomach. However, technical success rates of fluoroscopic placement have been reported to be similar to those of endoscopic placement.3–21 In some cases, fluoroscopic stent placement is successful after failed endoscopic placement. The delivery system of a covered stent is too large to place through the endoscope channel. In addition, accurate stent deployment is performed mainly under fluoroscopic monitoring. Therefore, the techniques used often depend on the referral pattern and available local expertise rather than superiority of techniques. The procedure can be performed on a conventional fluoroscopic table or within a vascular-interventional suite. Equipment allowing tilting of the table is desirable. Because of prolonged obstruction, the stomach is usually distended and elongated, which may make catheterization of lesions difficult or even impossible. Therefore, decompression of the stomach with a nasogastric tube for 1 to 2 days before the procedure is mandatory. The procedure is generally performed with the patient under conscious sedation and continuous monitoring of vital signs and oxygen saturation. After anesthetizing the pharynx with topical spray, an angiographic catheter with a guidewire is passed perorally into the stomach or duodenum near the obstruction (Fig. 130-3). A curved 100-cm-long angiographic catheter, such as a 5F multipurpose (Terumo Medical Corp., Tokyo, Japan) or Head Hunter catheter (Wilson-Cook), and a 260-cm, 0.035-inch floppy-tipped guidewire (Radiofocus [Terumo]) are most commonly used. A limited amount of iodinated contrast material is injected through the catheter to identify the proximal extent of the stricture. The catheter is manipulated through the obstruction with standard catheter and guidewire techniques. However, when the patient has a markedly distended stomach, manipulating the obstruction may prove difficult because the catheter and guidewire are frequently looped along the greater curvature of the stomach. A guiding sheath may be helpful to prevent looping of the guidewire in these cases.22 In addition, gastric decompression for several days before the procedure can minimize this problem. After the catheter is advanced as far distally as possible, contrast medium is injected again to demonstrate the distal margin of the obstruction. With the use of a recently developed GI catheter (Song-Lim [S&G Biotech]), the contrast medium can be injected without removing the guidewire from the catheter (Fig. 130-4).23 The guidewire is exchanged for a long stiff wire (260-cm Amplatz Super Stiff wire [Boston Scientific], Lunderquist Extra Stiff guidewire [Cook Medical, Bloomington, Ind.]). Sometimes the exchange-length guidewire is too short for the procedure because of marked looping of the catheter in the distended stomach. In these cases, use of a 500-cm-long Amplatz Super Stiff wire may solve the problem. Once an exchange stiff wire is placed, looping of the wire is likely to be relieved, and a straight access route can be established. As an alternative to the peroral approach, access to the stomach can be achieved by percutaneous gastrostomy (Fig. 130-5). This access allows placement of a stiff sheath to provide greater catheter and wire control. Consequently, such direct access allows successful catheterization in virtually all cases.5,16 However, percutaneous gastrostomy is an invasive procedure that has its own associated risks and complications, so it should be reserved as a last option. After placement of the stent by means of the gastrostomy route, a gastrostomy tube is left in place until a mature tract develops, usually 10 days after the procedure. Placement of duodenal and biliary stents from a transhepatic approach has also been reported.24 This alternative route is useful in patients with complex surgical anastomoses when peroral stent placement is not possible.
Intervention for Gastric Outlet and Duodenal Obstruction
Indications
Contraindications
Equipment
Technique
Technical Aspects
Peroral Stent Placement
Fluoroscopic Guidance
Transgastrostomy Stent Placement

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree