CHAPTER 24 Interventional oncology
The number of patients diagnosed with cancer each year is growing. In 2002, there were 10.9 million new cases worldwide, 6.7 million deaths, and 24.6 million people alive with cancer (within 3 years of diagnosis).1 The most commonly diagnosed cancers are lung, breast, and colorectal, and the most common causes of cancer death are lung, stomach, and liver cancer.2 Primary liver cancer is the sixth most common cancer worldwide and the incidence of primary liver cancer is increasing. However, metastatic tumors to the liver are more common than primary tumors.3 Significant improvements in chemotherapy and surgical options as well as the advancement of endovascular and image-guided treatments and the expanded role of interventional oncology have led to increased survival in these patients.
Liver
Hepatocellular carcinoma and other primary liver tumors (online cases 25, 58, 79, and 101)
There were an estimated 17,550 new cases and 15,420 deaths due to primary liver cancer in 2005 in the United States. These numbers yield an incidence of 5.4 per 100,000.4 Hepatocellular carcinoma (HCC) accounts for the vast majority of primary liver cancer in the United States and around the world. Other less common primary liver cancers include cholangiocarcinoma, fibrolamellar carcinoma, hepatoblastoma, and angiosarcoma of the liver.
Etiology and natural history
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are the main risk factors for HCC.5 Risk factors for viral hepatitis include contaminated blood transfusions, intravenous drug use, needle sharing, and unsafe sexual practice. HCC develops from HBV and HCV after a latency period of one to three decades.6 Other risk factors for developing HCC include alcohol abuse, hemochromatosis, aflatoxin exposure, diabetes mellitus, and nonalcoholic fatty liver disease. Studies have demonstrated a dose-response relationship between alcohol consumption and the risk for HCC development.7 As with alcohol, most risk factors are linked to HCC through the development of cirrhosis. However, up to one fourth of HCC cases arise in nonfibrotic livers.8 If untreated, the 1-, 2-, and 3-year survival rates in patients with HCC is 54%, 40%, and 28%, respectively.9
Clinical features
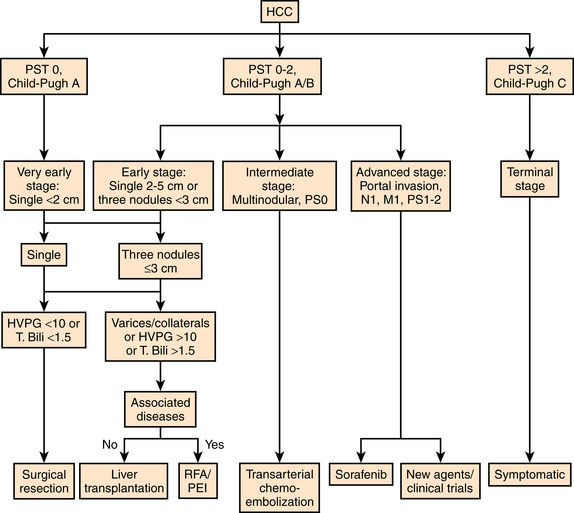
Patients with HCC often have symptoms related to their underlying liver disease as well as their malignancy. The prognosis of solid tumors is generally related to tumor stage at presentation and thus tumor stage ultimately guides treatment decisions. However, assessment of clinical outcome is particularly complex in HCC because the underlying liver function also affects prognosis. Historically, HCC was classified by the traditional TMN staging system based on pathologic findings without consideration of underlying liver function.10 The Okuda Staging System takes tumor size and liver function into account; however it is unable to adequately stratify patients with early or intermediate stage disease11 (Table 24-1). The Child-Pugh and the Model for End-stage Liver Disease (MELD) assessment classifications only consider liver function. The Barcelona Clinic Liver Cancer (BCLC) staging system is based on the combination of data from several independent studies representing different disease stages and treatment strategies.12–14 This classification scheme comprises four stages that match the best candidates for the best therapies and includes variables related to tumor stage, liver functional status, physical status, and cancer-related symptoms (Fig. 24-1).
Table 24-1 Okuda Staging System
| Score | |||
|---|---|---|---|
| Criteria | 0 | 1 | |
| Tumor size | <50% of liver | >50% of liver | |
| Ascites | Absent | Present | |
| Albumin (g/dL) | ≥3.0 | <3.0 | |
| Bilirubin (mg/dL) | <3.0 | >3.0 | |
Stage I, score 0; stage II, score 1 or 2; stage III, score 3 or 4.
Imaging
Ultrasound
Ultrasound is an excellent and inexpensive screening tool for HCC diagnosis, but it is highly dependent on the operator. HCC usually appears as a round or oval mass with sharp boundaries. HCC may exhibit a hypoechoic, isoechoic, or hyperechoic appearance with respect to the surrounding liver parenchyma. A small hyperechoic HCC may be indistinguishable from hemangioma.15 The reported sensitivity of conventional ultrasound in the detection of HCC ranges from 35% to 84%.16 Using Doppler or power Doppler sonography to evaluate for hypervascularity or portal venous invasion can increase the accuracy in the detection of HCC; however, detection of smaller lesions (≤1 cm) remains problematic.
Computed tomography
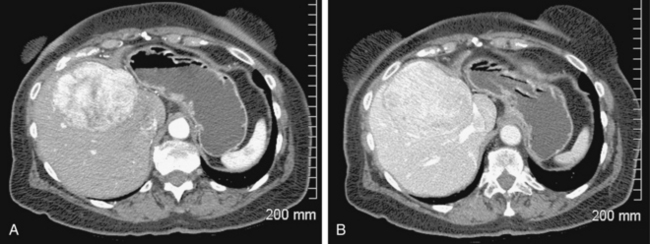
Computed tomography (CT) is a common imaging modality used for detection and diagnosis of HCC. A three-phase technique including noncontrast, early arterial, and portal venous (PV) inflow imaging is standard of care.17The value of adding delayed scans (e.g., 3 to 5 minutes after contrast injection) to depict pathologic tumor washout has been demonstrated.18 HCC typically appears as a hypervascular lesion in the early arterial phase with washout to an isodense or hypodense appearance in the portovenous phase (Fig. 24-2). In addition to the number and size of lesions, patency of the portal vein and the presence of extrahepatic disease can be assessed. According to American Association for the Study of Liver Disease (AASLD) and European Association for the Study of the Liver (EASL) guidelines, hypervascularity with washout in the PV phase on two imaging examinations for small lesions (1 to 2 cm) or one imaging examination for larger lesions (>2 cm) is diagnostic of HCC. A biopsy is not necessary in these cases. However, for small lesions (<1 cm), it is difficult to distinguish between regenerative nodules and HCC. In addition, arteriovenous shunts may mimic small HCCs.19
Magnetic resonance imaging
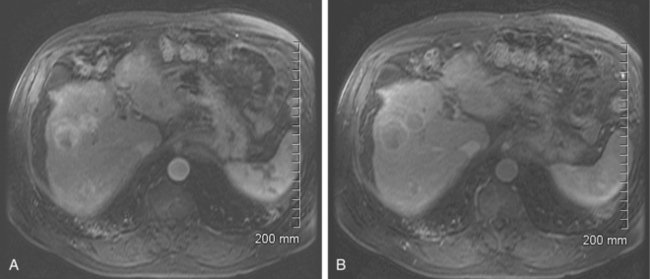
As with CT, hypervascularity with delayed washout in a cirrhotic liver are characteristics most consistent with HCC (Fig. 24-3). As with CT and ultrasound, the detection and characterization of lesions smaller than 1 cm remains problematic. However, with magnetic resonance imaging (MRI), the criteria for evaluation of focal lesions in cirrhotic liver can be expanded from vascularity alone to cellular density and tissue composition by means of precontrast sequences and diffusion-weighted MRI, to the presence of Kupffer cells by means of uptake of superparamagnetic particles of iron oxide and to the integrity of hepatocellular function and biliary excretion by means of hepatobiliary contrast agents.20 Whereas the use of these agents is not standard practice, they may prove to increase the sensitivity and specificity of lesion detection and diagnosis of HCC with MRI, especially in the case of lesions that are less than 1 cm in size. Burrel and colleagues reported a sensitivity of 100% for lesions larger than 2 cm, 89% for lesions between 1 and 2 cm, and 34% for lesions smaller than 1 cm.21
Treatment
For patients diagnosed with HCC, there are many treatment options available that will potentially have a positive impact on survival.22 To achieve the best outcome requires careful selection of candidates for each treatment option. Because this is a complicated disease process and there are multiple potentially beneficial therapies, patients with HCC should be referred to a multidisciplinary team of doctors, including hepatologists, oncologic surgeons, transplant surgeons, interventional radiologists, and medical oncologists, among others.
Medical therapy
Historically, no systemic chemotherapy has improved survival in patients with advanced HCC.23,24 However, sorafenib (Nexavar) is an oral tyrosine kinase inhibitor agent that has shown some survival benefit in patients with advanced HCC. Sorafenib inhibits tumor cell proliferation and angiogenesis. In a recent multicenter, phase III clinical trial with more than 600 patients, median survival in patients treated with sorafenib was nearly 3 months longer than those treated with placebo.25 The most common side effects of sorafenib in the trial were diarrhea, weight loss, hand-foot skin reactions, and alopecia.
Surgical therapy
For treatment with curative intent, surgical resection (partial hepatectomy or liver transplantation) has been at the forefront of treatment options for patients with HCC (see Fig. 24-1). However, HCC often occurs in patients with cirrhosis, which increases the risks associated with operation. Advances in perioperative care, patient selection, and radiologic assessment have led to decreased morbidity and mortality associated with surgical resection.26 Some of the most important factors in determining whether surgery is an option for patients with HCC include performance status, hepatic function and reserve, extent and location of tumor, and the presence of vascular invasion. Some of the surgical options include tumor excision, trisegmentectomy, and right or left hepatectomy.
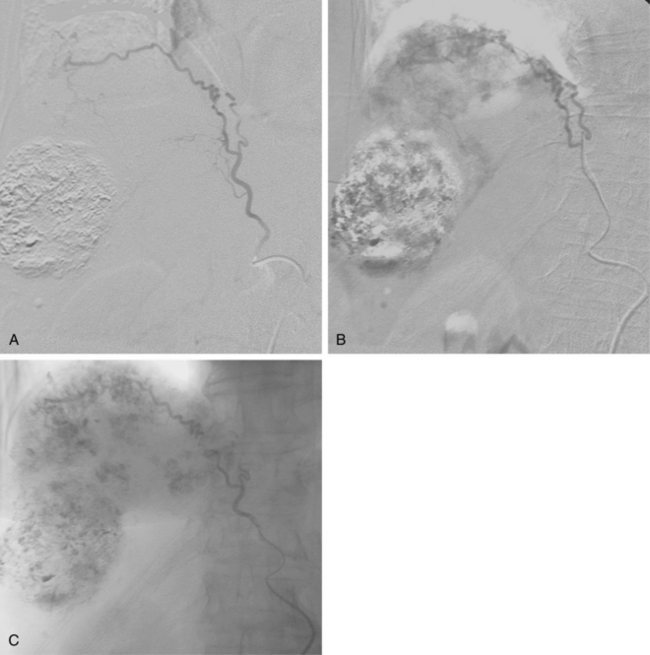
To improve results following hepatectomy, portal vein embolization may be necessary. In cirrhotic patients in whom resections to remove more than two functional segments are planned, portal vein embolization should be considered as a preoperative adjunct to induce hypertrophy of the liver remnant.27Portal vein embolization is a technique used to occlude portal inflow to the portion of the liver that is going to be removed, inducing hypertrophy in the portion of the liver that will be left behind, known as the liver remnant, over a period of 3 to 4 weeks (Fig. 24-4). This technique increases the ability to resect the liver safely.28
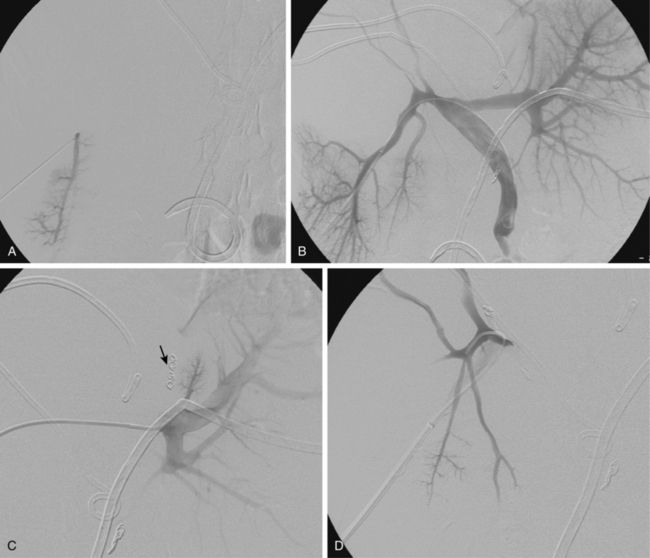
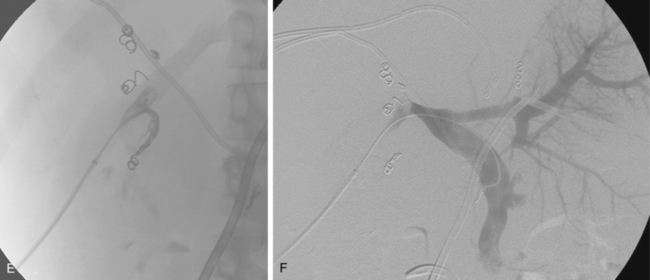
Figure 24-4 Portal vein embolization before right trisegmentectomy. A, Access is gained to a right portal vein branch with sonographic guidance. B, A vascular sheath has been placed, followed by portography, which shows distortion of right portal branches by tumor. C, Portal branches to segment IV have been embolized (arrow). D and E, Right portal venous branches are engaged and then occluded. F, Final portogram shows complete obstruction of branches to liver segments IV-VIII.
Transplantation represents effective treatment for the underlying liver disease as well as the cancer. However, the surgical risks of graft failure and infection, the shortage of donor organs leading to a long waiting list, and the risks associated with lifelong immunosuppression therapy can be seen as disadvantages.29 The most widely accepted Milan criteria include patients with a solitary tumor of 5 cm or less in diameter or patients with no more than three tumors, each 3 cm or less in diameter, and no invasion of major blood vessels, lymph nodes, or extrahepatic sites.30 Using these criteria, 4-year survival rates of up to 85% have been reported. Slightly expanded thresholds such as the UCSF criteria (single lesion ≤6.5 cm or two lesions ≤4.5 cm with total tumor diameter ≤8 cm) have also shown favorable 1-and 5-year survival rates of 92.1% and 80.7%, respectively.
Percutaneous therapy
Transcatheter arterial chemoembolization and bland embolization
Liver tumors, both primary and metastatic, receive their blood supply predominantly from the hepatic artery.31 Because the normal liver has dual blood supply (75% portal vein, 25% hepatic artery), chemoembolization delivers localized treatment to tumors without significant damage to the adjacent parenchyma. Chemoembolization also delivers higher doses of chemotherapeutic agents to liver tumors than does systemic therapy. In addition, the embolization component of chemoembolization prolongs the dwell time of the chemotherapeutic agents within the liver, thereby limiting systemic toxicity.32 Regarding transcatheter arterial chemoembolization (TACE), variations in protocol exist, including the chemotherapeutic used, use of lipiodol, and the type of embolic agent used to decrease blood flow. In the United States, doxorubicin, cisplatin, and mitomycin are the drugs most often used.33 Our protocol for a standard chemoembolization is 50 mg doxorubicin, 10 mg mitomycin, and 100 mg cisplatin. Lipiodol is an iodized ester from poppyseed oil. When it is injected into the hepatic artery, it is cleared from normal hepatic tissue but accumulates in tumor cells because of the absence of Kupffer cells.34
Patient selection
Several factors are important when determining whether a nonoperable patient is an appropriate candidate for chemoembolization. In patients with advanced liver disease, treatment-induced liver failure may offset the antitumoral effect or survival benefit of the intervention.35 Therefore some of the predictors of outcome are related to neoplastic burden: tumor size, number of tumors or percentage of liver involved with tumor, and extent of vascular invasion, if present. The degree of liver dysfunction should also be evaluated. This can be accomplished with the MELD assessment. Exclusion criteria base on specific laboratory values have not been definitively established. In addition, the performance status of the patient is vital in determining whether a patient will be able to tolerate chemoembolization as a treatment for the liver tumor. Two scales of performance status include the Karnofsky index and the Eastern Cooperative Oncology Group (ECOG) toxicity and response criteria (Tables 24-2 and 24-3). The best candidates are patients with liver dominant disease (no significant extrahepatic disease), preserved liver function, and a performance status of ECOG 0 or 1.
Table 24-2 Eastern Cooperative Oncology Group Performance Status
| Grade | ECOG |
| 0 | Fully active, able to carry on all predisease performance without restriction |
| 1 | Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature (e.g., light house work, office work) |
| 2 | Ambulatory and capable of all self-care but unable to carry out any work activities. Up and about more than 50% of waking hours |
| 3 | Capable of only limited self-care, confined to bed or chair more than 50% of waking hours |
| 4 | Completely disabled. Cannot carry on any self-care. Totally confined to bed or chair |
| 5 | Dead |
Table 24-3 Karnovsky Performance Status Scale
| 100% | Normal, no complaints, no signs of disease |
| 90% | Capable of normal activity, few symptoms or signs of disease |
| 80% | Normal activity with some difficulty, some symptoms or signs |
| 70% | Caring for self, not capable of normal activity or work |
| 60% | Requiring some help, can take care of most personal requirements |
| 50% | Requires help often, requires frequent medical care |
| 40% | Disabled, requires special care and help |
| 30% | Severely disabled, hospital admission indicated but no risk of death |
| 20% | Very ill, urgently requiring admission, requires supportive measures or treatment |
| 10% | Moribund, rapidly progressive fatal disease processes |
| 0% | Death |
In addition to prolonging survival and/or treating symptoms in unresectable patients, locoregional therapies have been shown to maintain patients on the transplant waiting list as well as downstage the disease in some patients.36
Absolute contraindications for chemoembolization include intractable systemic infection and extensive extrahepatic disease. Relative contraindications include portal vein invasion, the presence of encephalopathy, and unrelieved biliary obstruction. Several studies have demonstrated the safety and effectiveness of TACE in patients with portal venous invasion when chemoembolization is performed in a subselective manner.37,38 Other relative contraindications include uncorrectable coagulopathy, significant arteriovenous shunting, and significant renal insufficiency. These are relative contraindications because these may be correctable or the treatment regimen may be altered to accommodate the abnormality. For example, in the case of a patient with angiographically visible shunting within the tumor, a bland embolization could be performed before chemoembolization in order to reduce the shunt and ensure adequate treatment of the tumor.
Technique
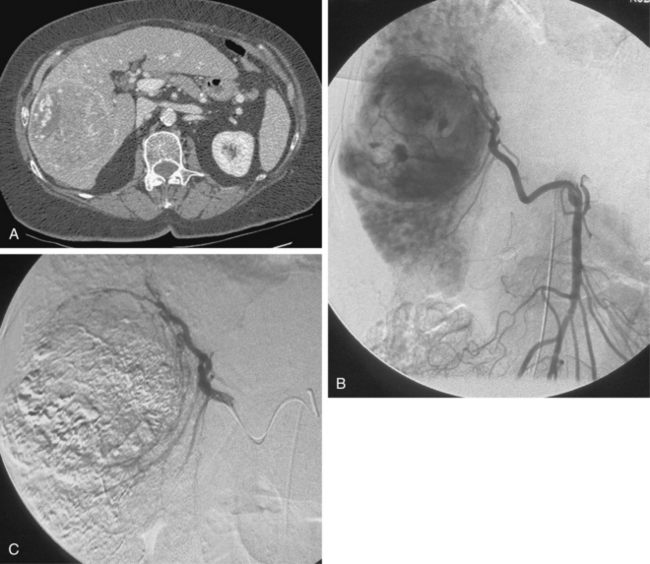
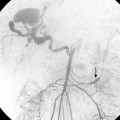
Patients receive preprocedure hydration as well as antiemetics and steroids (Box 24-1). Preprocedure antibiotics are altered for the patient who has a compromised sphincter of Oddi from prior surgery or biliary stent placement. These individuals are at increased risk for infection and abscess formation following chemoembolization.39,40 For these patients, we prescribe moxifloxacin 400 mg daily for 10 days before the procedure. Diagnostic arteriography of the celiac and superior mesenteric arteries is essential to identify the arterial supply to the tumor as well as aberrant vessels supplying the tumor or extrahepatic structures. The arteriogram should be carried out to the venous phase to evaluate the patency of the portal vein. A microcatheter is then used to selectively catheterize the tumor arteries. It is also important to evaluate and identify any arteriovenous shunting, which has been reported to occur in 31% to 63% of cases between second order branches41 (Fig. 24-5). Practice patterns vary as to whether a superselective, segmental, or lobar approach is performed (Fig. 24-6). However, treatment of the entire liver in one session is associated with increased mortality.42 It may be necessary to evaluate extrahepatic arterial supply to the tumor as well, such as the inferior phrenic artery. In a retrospective study of more than 2300 procedures, TACE through an extrahepatic collateral was considered in 25% of cases43 (Fig. 24-7). A variety of TACE protocols exist and there is great variability in the amount and type of the chemotherapeutic agents used, type and amount of embolization agents, and sequencing of embolization.
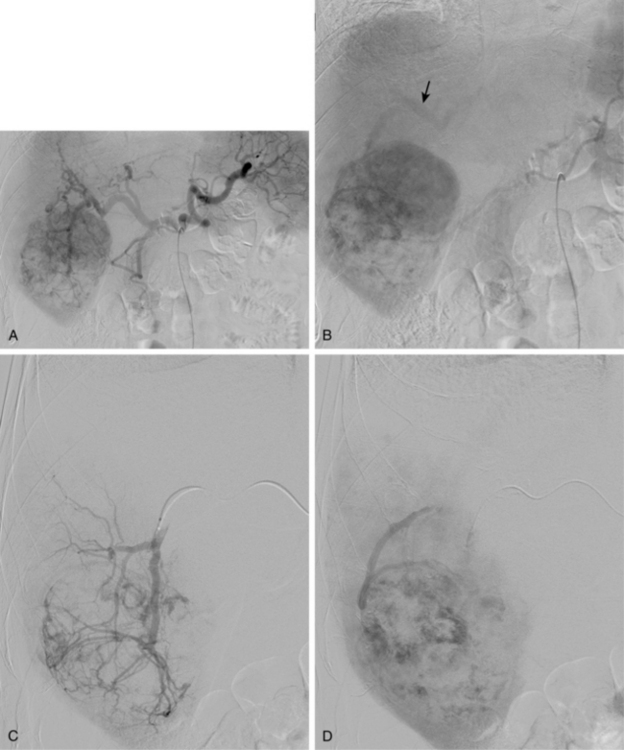
Figure 24-5 Hepatocellular carcinoma. A and B, Celiac arteriogram demonstrating lesion within the right hepatic lobe. Notice the early appearance of the portal vein (arrow). C and D, Subselective arteriogram demonstrating arterial portal shunting within the tumor.
Results
Chemoembolization has been shown to be a safe and effective treatment for selected patients with HCC. Two prospective, randomized trials demonstrating the survival benefits of chemoembolization in HCC have been published. A study by Lo and colleagues44 compared patients treated with chemoembolization with those given supportive care and demonstrated improved survival at 1, 2, and 3 years. Another study by Llovet and colleagues compared chemoembolization and hepatic arterial embolization with supportive care and demonstrated a survival benefit in the embolization treatment groups.45
Complications
Postembolization syndrome is characterized by abdominal pain, fever, anorexia, nausea, and fatigue. The cause is not completely understood but several theories exist including distention of the liver capsule, tumor necrosis, and ischemia of liver parenchyma.46 The severity of symptoms is variable. They may last as long as 10 days and may occasionally require an extended hospital admission. The postembolization syndrome occurs in up to 90% of patients following chemoembolization and for that reason is considered a side effect rather than a complication.47
Nontarget embolization, bile duct injury, and hepatic failure are the potentially most severe complications following chemoembolization (Table 24-4). Nontarget embolization is defined as the inadvertent distribution of chemotherapeutic agents into unintended territories. Diagnostic arteriography prior to infusion of chemotherapeutic agents is vital to identify any extrahepatic arterial variants in the treatment region. Nontarget embolization of a gastric artery or the gastroduodenal artery can cause gastrointestinal ischemia. If TACE cannot be performed distal to the extrahepatic artery, embolization of the extrahepatic artery (with coils, if safe) can be effective in preventing complications related to nontarget embolization.31 Hepatic failure is more likely to occur in patients with impaired hepatic function prior to treatment. Acute liver failure has been reported in up to 2.3% of patients following chemoembolization.48 In contrast to normal hepatic parenchyma, intrahepatic bile ducts do not have dual blood supply. Rather, they are fed exclusively from hepatic arterial branches that give off a vascular plexus around the bile ducts.49 Therefore, ischemic bile duct injury can occur following chemoembolization.
Table 24-4 Major Complications of Hepatic Arterial Chemoembolization
| Major Complication | Reported Rate (%) | Suggested Threshold (%) |
|---|---|---|
| Liver failure | 2.3 | 4 |
| Postembolization syndrome requiring extended stay or readmission | 4.6 | 10 |
| Abscess with functional sphincter of Oddi | 1.0 | 2 |
| Abscess with biliary-enteric anastomosis/biliary stent/sphincterotomy | 25.0 | 25 |
| Surgical cholecystitis | 1.0 | 1 |
| Biloma requiring percutaneous drainage | 1.0 | 2 |
| Pulmonary arterial oil embolus | 1.0 | 1 |
| Gastrointestinal hemorrhage/ulceration | 1.0 | 1 |
| Iatrogenic dissection preventing treatment | 1.0 | 1 |
| Death | 1.0 | 2 |
From Brown DB, Cardella JF, Sacks D, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol 2006;17:225.
Yttrium-90 radioembolization
Normal hepatic parenchyma is very sensitive to tumoricidal radiation doses. External beam radiation has had a limited role, in that as doses greater than 35 Gy have been shown to cause the development of radiation-induced liver disease. Radioembolization is defined as the intraarterial delivery of radioisotope-labeled particles that become embedded in the tumor preferentially to surrounding parenchyma because of differences in vascular supply.50 Yttrium-90 (90Y) is a pure beta emitter and decays to 90Zr with a physical half-life of 64.2 hours. The energy has a mean tissue penetration of 2.5 mm and a maximum penetration of 11 mm. There are two commercially available agents: SIR-Spheres (Sirtex Medical Ltd, Lane Cove, Australia) and TheraSphere (MDS Nordion, Ottawa, Canada). The 90Y is bound to either glass or resin microspheres and delivered into the hepatic arteries via a microcatheter. To optimize safety and efficacy, radiation dose planning and administration is modified on the basis of tumor and liver volumes. Flow dynamics cause the particles to travel preferentially to the tumor.
Patient selection
A Consensus Panel Report51 that was published in 2007 provides detailed guidelines in regard to patient selection for radioembolization. A summary of some general patient selection criteria and contraindications are included in Box 24-2. Because a small amount of adjacent liver will be affected by the radiation, sufficient hepatic reserve is required. Parameters of adequate hepatic reserve include lack of ascites, normal synthetic liver function (e.g., albumin >3 g/dL) and normal total bilirubin (<2 mg/dL).52 Performance status is also critical in patient selection and good candidates have a performance status of ECOG 0-2. An absolute contraindication to radioembolization is the predicted administration of a dose of at least 30 Gy to the lungs in a single treatment or greater than 50 Gy as a cumulative dose on multiple treatments53 (see later discussion).
Technique
Starting a 90Y program is complicated. The treatment itself consists of two phases. In the first phase, patients undergo an angiogram to map the arterial system and embolize any vessels that would allow the microspheres to enter the gastrointestinal tract (i.e., skeletonize the hepatic circulation).51 A superior mesenteric angiogram is performed to detect replaced or accessory hepatic arteries. In addition, the arteriogram is carried out to the venous phase to evaluate the status of the portal vein. A celiac arteriogram and subsequent selective hepatic angiography is performed to define the hepatic and tumoral anatomy and to evaluate for anatomic variants (Fig. 24-8). This step is critical in that the presence of unrecognized collateral vessels with consequent infusion of radioactive microspheres could lead to gastrointestinal ulceration, pancreatitis, and irradiation of other nontarget sites.54 For this reason, aggressive prophylactic embolization of these aberrant vessels before therapy is essential. The gastroduodenal artery (GDA) is virtually always embolized to its origin with microcoils. Some of the relatively common vessels of interest include the right gastric, esophageal, accessory left gastric, falciform accessory phrenic, and supraduodenal or retroduodenal arteries. Once the arterial anatomy has been evaluated and the aberrant gastrointestinal supply, if present, is embolized, the degree of lung shunting through the tumor must be estimated. To this end, 5 mCi of 99m
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree