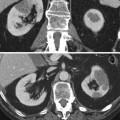
Fig. 1
Spot fluoroscopic image of the left kidney for lithotomy. Image shows left staghorn calculus (black arrow), a ureteral stent had been placed (white arrow), and a needle (arrowhead) is being advanced into midpole calyx after retrograde injection of contrast to opacify the renal collecting system
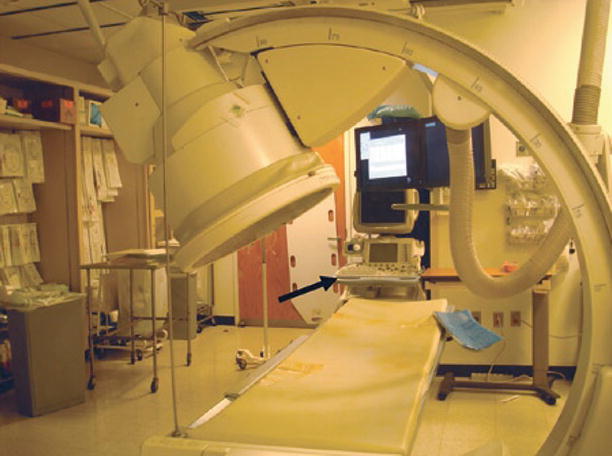
Fig. 2
Photograph of interventional suite showing the ability to tilt image intensifier to aid in interventional procedures involving the urinary system. A nearby ultrasound machine (arrow) is also always available for use during interventional procedures
2.1.2 Ultrasonography
Ultrasound has been used diagnostically to evaluate the kidneys. Typically, a 2–4 MHz transducer is used to image the kidneys via a flank approach. Sonography can evaluate the size of the kidneys, the appearance of the cortex, and the presence of hydronephrosis and can evaluate for the presence of renal cysts, stones, or masses (Fig. 3).


Fig. 3
Sagittal ultrasound of the kidney showing moderate hydronephrosis. The cortex is preserved. The hydronephrosis (arrow) can easily be targeted by an access needle for nephrostomy tube placement
In interventional uroradiology, ultrasound is used for focal or nonfocal renal biopsies, guiding initial access for nephrostomy tube or nephrolithotomy needle placement, and for suprapubic tube placement. Advantages of ultrasound include real-time visualization, portability, and lack of ionizing radiation. Limitations include the need for specialized technical skills to properly scan the renal collecting system and guide the proper placement of needles and/or catheters. Another limitation is the potential for poor visualization of the kidneys due to overlying bowel gas or thickened subcutaneous and retroperitoneal adipose tissues.
2.1.3 CT
Increasingly, CT scanners are becoming available exclusively for interventional procedures. Modifications to a standard diagnostic CT, including the addition of CT fluoroscopy, larger diameter gantry and the availability of multiplanar reformatting (Fig. 4), have promoted the use of CT for image-guided procedures. CT is used for renal biopsies, renal tumor ablations, drainage of renal or perirenal collections, and, on a few occasions, percutaneous nephrostomy (Egilmez et al. 2007). The advantages of CT include 2D visualization of internal organ structures and improved tissue contrast over ultrasound and fluoroscopy. Limitations include radiation to the patient and to the radiologist, if CT fluoroscopy feature is used.
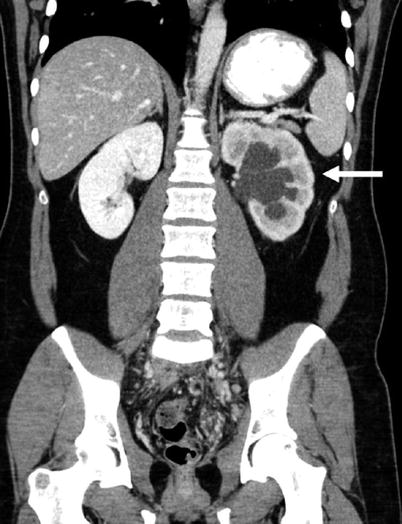

Fig. 4
Coronal reformatted contrast-enhanced CT showing left hydronephrosis and delayed left renal nephrogram (arrow)
3D cone-beam computed tomography (CBCT) is a novel CT guidance technique, which is in the experimental phases. It uses a combination of cone-beam CT and real-time fluoroscopy in the angiography suite. Early studies promisingly showed that it could allow safe and accurate biopsy of focal small renal lesions, especially in hard-to-reach anatomical locations or lesions invisible on ultrasound (Braak et al 2012).
2.1.4 Interventional MRI
Interventional MRI is being used for image-guided procedures (Fennessy et al. 2008) at a few centers worldwide. It has been used to perform cryoablation of renal tumors (Silverman et al. 2005). The advantages of interventional MRI include superior tissue contrast; multiplanar imaging, which allows access to lesions that require a steep angulation; and no radiation. Limitations include limited availability of interventional MRI equipment, need for MR-safe interventional equipment, needle artifact, and cost.
2.2 Interventional Equipment Tools
In addition to advances in imaging modalities, advances in the design of needles, guidewires, and catheters have pushed the field of interventional uroradiology forward.
2.2.1 Needles
Many interventional uroradiology procedures can be performed due to the availability of small-bore 17–22-gauge needles. These needles are available from sizes varying from 6 to 25 cm and allow percutaneous access to the kidney or renal collecting system with minimal damage to the surrounding structures (Lee 2004a). Needles are used for renal biopsies, access to renal or perirenal fluid collections, and access to the renal collecting system for nephrostomy tube placement or for lithotomy.
Coaxial needles may be used to obtain multiple samples through a single puncture (Fig. 5). The advantages are increased patient tolerability for the procedure and decreased potential for tumor seeding along the needle tract as reported in a study of breast biopsies using coaxial needles by Helbich et al. (1997).
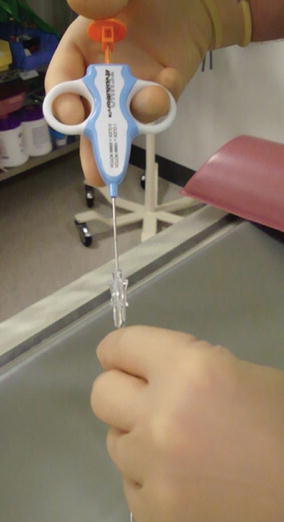

Fig. 5
Photograph of a 17-gauge Temno coaxial needle system (Cardinal Health Vaughan, ON) used for our focal renal biopsies
2.2.2 Guidewires
Guidewires are useful tools in image-guided urological procedures. Wires used for interventional procedures vary from 0.018 to 0.038 in. and have various degrees of torquability (Fig. 6). The tips of the guidewires can be curved as in a 3 J-wire or straight. Commonly used wires in interventional uroradiology include stainless steel 0.018 wire for initial access to the renal collecting system, 3 J-wire for manipulation within the renal pelvis and ureter, and a 0.038 in. stiff wire for guiding placement of dilators and catheters. The use of hydrophilic coatings on wires has allowed interventional radiologists to slide into extremely small-radius curves that were difficult or impossible to access before such materials existed.
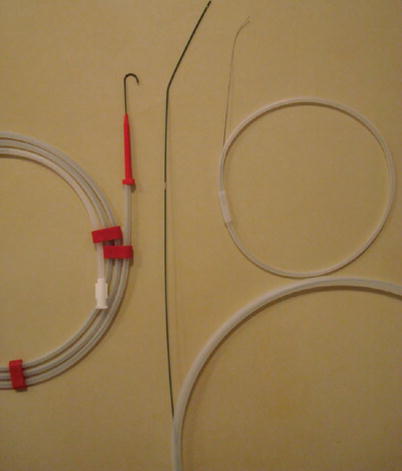

Fig. 6
Photograph of 0.018 in., 3 J, and stiff 0.038 in. wires used for interventional procedures
2.2.3 Catheters
Advances in materials used for catheter development, including copolymers of polyurethanes and silicone, have led to the creation of soft catheters that resist occlusion by adherent materials, are well tolerated, and have large internal lumen diameters for a small outer diameter. This has made long-term indwelling catheter use comfortable for patients. Commonly used catheters in interventional uroradiology vary from 7 French to 14 French. Various configurations of catheters exist with various multiple-side-hole configurations and various pigtail locking configurations (Fig. 7). Catheters are used for drainage of renal or perirenal collection, nephrostomy tubes, ureteral stents, and suprapubic tubes. Many of these catheters can be placed into the final position using a guidewire or can be directly punctured into place with the help of a sharp-tipped internal stiffener.
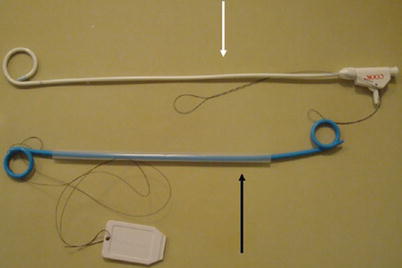

Fig. 7
Photograph of pigtail nephrostomy tube (white arrow) and double pigtail ureteral stent (black arrow)
2.2.4 Stents and Balloon Catheter
Ureteral stents are thin soft tubes that are placed temporarily in the ureter to maintain ureteral patency for urine flow in case of ureteral obstruction. Several factors are important for choosing the apau propriate stent for each case including length, anchoring system (double-J stent or tail stent), and material of stent (silicone, polyurethane, copolymers, and metallic stents).
There are various types of balloon catheters. These can be used prior to stone manipulation or stenting, ureteroscopy, and dilating the intramural ureter.
3 Renal Biopsy
3.1 Indications
Image-guided focal renal biopsies are performed to diagnose renal cell carcinoma and renal metastatic disease or to evaluate for renal lymphoma (Wood et al. 1999). Although some individuals avoid biopsy of renal cell cancer due to risk of tumor seeding, the incidence of tumor seeding is less than 0.01 % (Lane et al. 2007). Alternatively, biopsy of lesion suspected to be renal cell carcinoma allows for a definitive diagnosis prior to ablation or operative nephrectomy and can aid in subclassifying the type of cancer for surveillance and future treatment (Wood et al. 1999). A study advocating renal biopsies prior to ablation identified that 37 % of renal masses referred for ablation were benign renal masses (Tuncali et al. 2004).
Nonfocal renal biopsies are performed to diagnose and make a decision about the management of some diffuse renal parenchymal diseases like glomerulopathies, tubulointerstitial nephropathies, or renal transplant rejection. There is no absolute contradiction for renal biopsy. Relative contraindications are uncontrolled coagulopathy and uncontrolled hypertension (Lane et al. 2008), (Khati et al. 2011; Sharma et al. 2010).
3.2 Preparation
The preparation to perform a renal biopsy includes the following: (1) Review of prior imaging to identify the kidney, area of abnormality, and identify adjacent critical organs such as the pleura, liver, spleen, colon, major vessels, or pancreas. (2) Review of coagulation parameters. At our institution, we use an international normalized ratio (INR) ≤1.5 and a platelet count ≥50,000 μl (mcL) as cutoff levels for performing renal biopsies. Holding anticoagulation medications including warfarin, heparin, aspirin, and fragmin and the administration of fresh frozen plasma or platelets may be used to address coagulation abnormalities. (3) Assessment of estimated glomerular filtration rate (eGFR) prior to a nonfocal renal biopsy is important as an index of severity of chronic kidney disease. Its formula is eGFR = 186 × (creatinine [micromole/L]/ 88.4)−1.154 × (age[year])−0.203 × (0.742 if female) × (1.210 if black). Uremia can lead to platelet dysfunction, which can increase the risk of bleeding after biopsy, and using desmopressin (DDAVP) can reduce bleeding complications. We typically administer DDAVP before nonfocal renal biopsies for patients who have an eGFR <30, which indicates stage 4–5 chronic kidney diseases (Manno et al. 2011; Whittier et al. 2011). (4) Assessment of the patient to tolerate laying prone, supine, or decubitus for an extended period (approximately 20–60 min) and to tolerate conscious sedation. (5) Antibiotic is not routinely administered before the renal biopsy. Focal and nonfocal renal biopsies can be performed under conscious sedation with midazolam and fentanyl or only with local anesthesia.
3.3 Technique
For CT-guided focal renal biopsies, patients are placed ipsilateral side down on CT, and a localizing grid is placed overlying the region of biopsy. Initial planning CT is acquired and the lesion to be biopsied and entrance sites are identified. The skin is prepped, draped, and anesthetized with 10 cc of 1 % lidocaine. A small skin incision is made and a 17-gauge core axial needle is advanced to the target. A subsequent CT confirms accurate targeting of the lesion. We use the 17-gauge coaxial needle, a 22-gauge Chiba (Dyna Medical Corp, London, ON) needle for fine needle aspirates, and an 18-gauge Temno biopsy (Cardinal Health, Vaughan, ON) for core biopsy samples. Subsequently, the needle is removed and a final post-procedure CT is performed to evaluate for perinephric hemorrhage or injury to adjacent organs.
In nonfocal renal biopsies under ultrasound guidance, patients are placed ipsilateral side up or prone. Initial ultrasound exam identifies the kidney and the entrance site; the skin is prepped and draped and anesthetized with 10 cc of 1 % lidocaine. The ultrasound probe is covered with sterile dressing. Under real-time sonography guidance, a 15-gauge needle is advanced into the lower pole cortex of the kidney. Needle trajectory should point away from the central hilar structures to avoid injury to the renal vasculature or pelvis (Fig. 8). We usually obtain one or two core samples, and then the biopsy needle is removed and a final post-procedure sonography is performed to evaluate for perinephric hemorrhage.
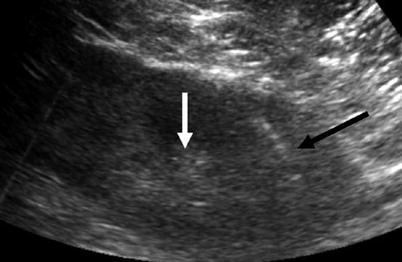

Fig. 8
Sagittal ultrasound of the left kidney shows biopsy needle trajectory extending into the left lower pole cortex (black arrow) away from the central renal hilar structures (white arrow)
3.4 Success Rate
Technical success rate (the ability to obtain tissue to submit for diagnosis) for image-guided percutaneous renal biopsy is greater than 80 %. According to a systematic review published about the diagnostic performance of renal biopsy for focal renal lesions, the overall sensitivity and specificity were 92.1 and 89.7 %, respectively, and the overall positive predictive value and negative predictive value were 95.7 and 82.0 %, respectively (Lane et al. 2008). Patients with diffuse kidney disease have a pathologic diagnosis of up to 100 % (Khati et al. 2011).
3.5 Complications
In recent studies minor complications from renal biopsies become less and are reported to be less than 1 %. The overall major morbidities like renal loss and mortality rate are exceedingly rare (0.03 %) (Smith 1991). Common complications of renal biopsies include transient hematuria or minor perinephric hemorrhage (Fig. 9). Transient hematuria and mild perinephric hemorrhage are not uncommon and they are usually self-limited and resolve within 24–48 h after the procedure. Severe perinephric hemorrhages with retroperitoneal blood extension into the pelvis or extensive hematuria with blood clots are rare. Patients with these complications usually require 24-h admission for serial checking of hematocrit and, if necessary, blood transfusion. On rare occasions, angiography is necessary to evaluate for pseudoaneurysms as a cause for ongoing persistent hemorrhage (Fig. 10). Other complications include infection and injury to adjacent organs including the lung (pneumothorax, hydrothorax, or hemothorax due to a transpleural access), liver, spleen, or colon. It is also recommended for all patients to seek medical care if they have gross hematuria or symptoms of hypotension or severe pain after discharge.
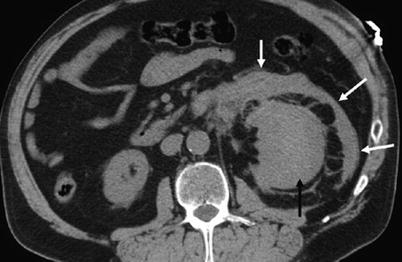
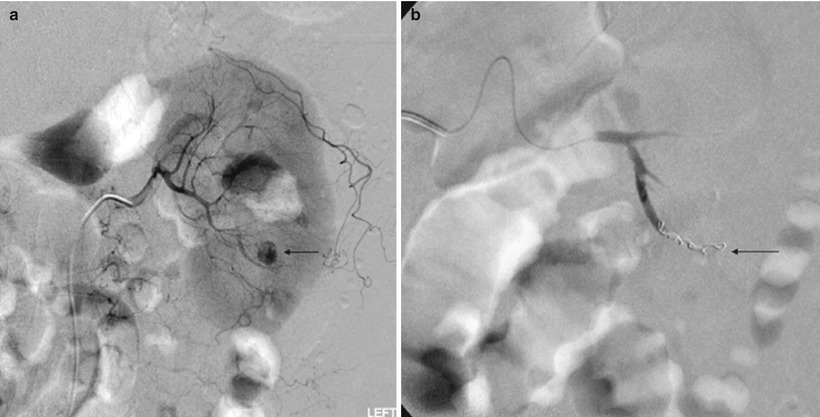
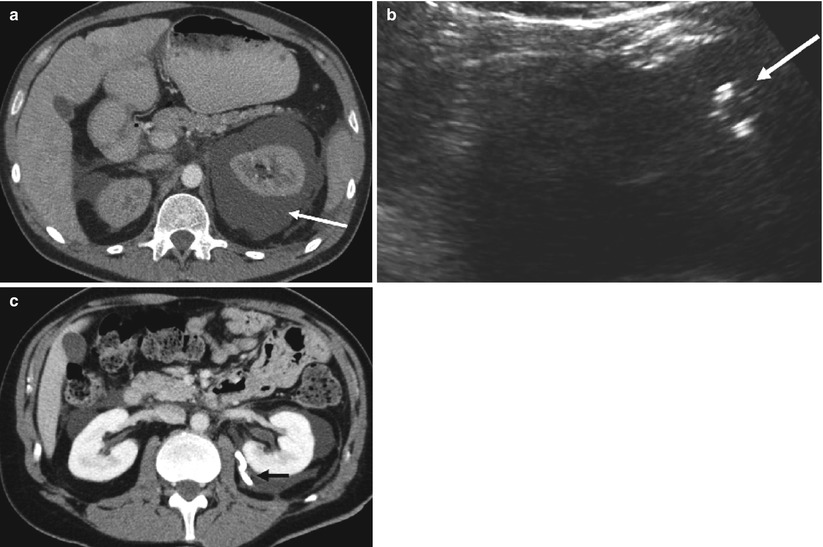

Fig. 9
Axial CT post-biopsy shows a large subcapsular (black arrow) and perinephric hemorrhage (white arrows)

Fig. 10
(a) Angiographic image of the left kidney shows a 2-cm pseudoaneurysm arising from lower pole branch vessel (arrow) after nonfocal renal biopsy. The patient had persistent hematuria and hematocrit drop prompting angiogram study. (b) Angiographic image of the left kidney postcoiling of the pseudoaneurysm shows coils within the left lower pole branch vessel (arrow) and the previously visualized pseudoaneurysm is no longer seen

Fig. 11
(a) Axial CT showing large left perinephric fluid collection (arrow). (b) Sagittal ultrasound of the perinephric fluid collection showing ultrasound-guided placement of pigtail catheter (arrow) into the collection. (c) Axial CT showing decompression of perinephric fluid after placement of pigtail catheter adjacent to the left kidney (arrow)
The risk of needle tract seeding of tumor is extremely low. Overall, needle tract seeding for any abdominal biopsy has been reported to be between 0.003 and 0.009 % (Smith 1991). A few cases of needle track seeding from renal mass biopsies have been reported in the literature (Gibbons et al. 1977; Kiser et al. 1986; Wehle and Grabstald 1986), and since 1994, there are no reported cases (Lane et al. 2008). The renal biopsy is covered with greater detail on chapter “The role of kidney biopsy in the diagnosis of renal disease and renal masses,” which is dedicated to this topic.
4 Percutaneous Nephrostomy
4.1 Indications
Percutaneous nephrostomy (PCN) tube placement is used to treat hydronephrosis due to urinary obstructions, pyonephrosis, and urinary diversion. It is also used to provide access for subsequent interventions in the renal collecting system or ureters. The relative contraindications of PCN are uncontrolled coagulopathy or hemodynamic instability.
4.2 Preparation
Review of prior ultrasound or CT imaging is useful to evaluate for the degree of hydronephrosis and potential cause for hydronephrosis and evaluate for ease of access to the kidney. Prophylactic antibiotics are given prior to the procedure.
4.3 Technique
Image-guided percutaneous nephrostomy tube placements are typically performed with a combination of both sonography and fluoroscopy (Dyer et al. 2002). Fluoroscopy can occasionally be used exclusively if there is a visible renal collecting system calculus or a retrograde ureteral stent that can be used to opacify the collecting system. Patients are placed prone oblique on the fluoroscopy table with the ipsilateral side facing the interventional radiologists. Patients are obliqued 20° to facilitate access to the posterior calix via the avascular plane of Brodel (Papanicolaou 1995; Scatorchia and Berry 2000).
At our institution we use either ultrasound (Fig. 12) or retrograde injection of contrast via stent to identify the renal collecting system. The skin is then prepped and draped and anesthetized with 1 % lidocaine. After initial needle placement, urine is aspirated from the collecting system and an equivalent amount of diluted contrast is instilled under fluoroscopy (Fig. 13a). If the initial needle axis is not adequate, defined as either an anterior calix, into the renal pelvis, or superiorly via a transpleural access, a second needle may be inserted under fluoroscopic guidance into a lower pole posterior calix (Fig. 13b). If the initial needle placement is adequate in a posterior calix below the 12th rib, a guidewire is inserted and subsequent dilatations are performed for eventual placement of a nephrostomy tube (Fig. 13c). After placement of a nephrostomy tube, urine is aspirated and sent to microbiology and a final nephrostogram is carefully performed confirming placement of the nephrostomy tube into the collecting system (Fig. 13d).
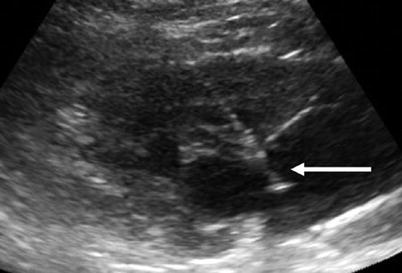


Fig. 12
Sagittal ultrasound of the kidney showing needle (arrow) guided into the renal collecting system for the placement of a nephrostomy tube

Fig. 13
(a) Fluoroscopic spot image after initial needle access under ultrasound guidance now used to carefully opacify the left renal collecting system (arrow). (b) Because initial needle access was above the 12th rib (black arrow), a second access was obtained using a sheathed needle directed into lower pole calyx under fluoroscopic guidance (white arrow). (c) Fluoroscopic spot image showing advancement of a curved wire (arrow) into proximal ureter via lower pole access. Access to the proximal ureter allows for a more stable purchase of the guidewire for subsequent dilations and nephrostomy tube placement. (d) Fluoroscopic spot image showing final placement of pigtail nephrostomy tube into renal collecting system via lower pole access (arrow)
4.4 Success Rate
If the collecting system is dilated, the technical success rate approaches 100 %. Even for nondilated systems, the success rate is as high as 80 % (Maher et al. 2000).