13 Intraspinal Masses
Approximately 15% of primary central nervous system (CNS) tumors are intraspinal. The ratio of intracerebral to intraspinal sites of occurrence for primary CNS tumors is approximately 10:1 for astrocytic tumors and about 3–20:1 for ependymomas, depending on the source.
As a general rule, the majority of intraspinal CNS tumors differ from most intracerebral CNS tumors in that they are benign and become symptomatic by compressing neural tissues, rather than by invading or destroying tissues.
Classification of Intraspinal Masses
Intraspinal masses can be divided into three groups according to the compartment that they occupy. This classification also provides a guide to differential diagnosis, based on the frequency of occurrence of particular tumor entities in different spinal compartments.
Intraspinal masses are classified by location into the following groups (Fig. 13.1):
• Extradural spinal masses
• Intradural extramedullary spinal masses
• Intramedullary spinal masses
Extradural masses. Extradural masses are the largest group, accounting for approximately 55% of spinal tumors. They may arise from the vertebral bodies or epidural structures, or they may invade the spinal canal secondarily from nearby structures (Fig. 13.2).
The largest group of extradural masses consists of metastases from the primary tumors listed below.
Bone tumors that arise from the vertebral bodies are among the most important primary extradural spinal tumors. They include:
• Chordoma
• Osteoid osteoma
• Osteoblastoma
• Aneurysmal bone cyst
• Vertebral hemangioma
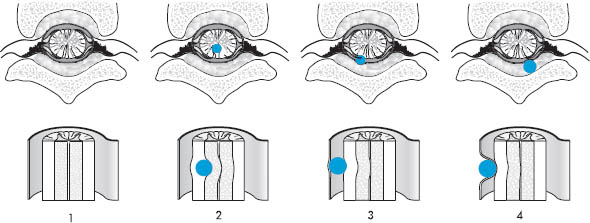
Fig. 13.1 Sites of occurrence of intraspinal masses.
1 Normal anatomy
2 Intramedullary mass
3 Intradural extramedullary mass
4 Extradural mass
Neurofibromas can also occur as primary extradural lesions.
Some tumors that are predominantly intradural can occur in the extradural compartment as well. For example, approximately 15% of spinal meningiomas present as extradural masses.
Intradural extramedullary mass. These masses make up approximately 40% of spinal tumors. They arise chiefly from the leptomeninges and nerve roots. The most important are:
• Meningioma
• Schwannoma
• Lipoma
• Neurofibroma
Approximately 4% of spinal metastases occur in the intradural-extramedullary compartment.
Intramedullary masses. Intramedullary neoplasms arise directly from the spinal cord, and make up approximately 5% of spinal tumors. Astrocytomas and ependymomas each account for approximately one-third of intramedullary tumors (up to 50%, according to some sources). The remaining third consists of various tumor entities that are rare individually, or occur infrequently as spinal neoplasms. They include:
• Glioblastoma
• Dermoid
• Epidermoid
• Teratoma
• Lipoma
• Hemangioma
• Hemangioblastoma
• Lymphoma
• Oligodendroglioma
Only about 2% of spinal metastases are intramedullary.
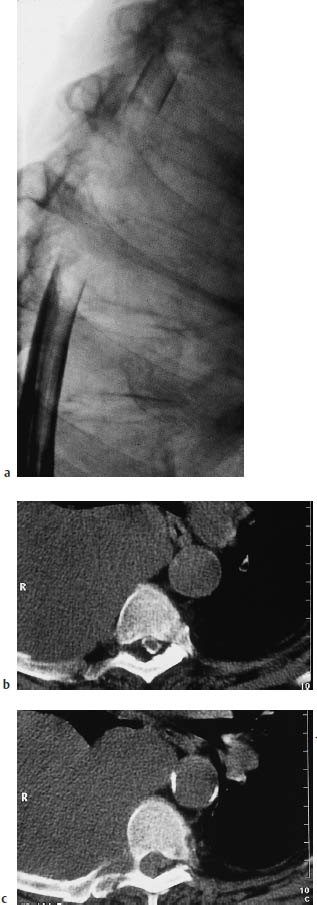
Fig. 13.2a–c Example of a paravertebral tumor invading the spinal canal secondarily. A right-sided pleural mesothelioma has invaded the spinal canal at multiple levels by contiguous spread through the neuroforamina. Compression of the dural sac and nerve roots appears as an elongated filling defect on myelography and postmyelographic CT.
a Myelogram.
b, c The postmyelographic CTs above (b) and below (c) the level of dural sac compression.
Metastases (Figs. 13.3–13.10)
Frequency: spinal metastases are the most common extradural mass, occurring in approximately 10% of cancer patients.
Suggestive morphologic findings: more than 90% of spinal metastases are extradural. Bone destruction is common. Lesions are usually multiple, and enhance after intravenous contrast administration.
Procedure: if metastasis is suspected, obtain primary contrast-enhanced computed tomography (CT) scans with a bone-window setting.
Other studies: Magnetic resonance imaging (MRI) can screen the entire spinal column for metastases. Compare with conventional radiography. Watch for contrast block in postmyelographic CT.
Checklist for scan interpretation:
 Number, location, and extent of lesions?
Number, location, and extent of lesions?
 Signs of resulting instability?
Signs of resulting instability?
 Cord compression?
Cord compression?
 Pathogenesis
Pathogenesis
Metastases are distinguished from other spinal masses by their propensity to occur in all three compartments. Most occur in the extradural compartment, however, and they most commonly involve the vertebral bodies (only 2–4% of metastases are intradural, 1–2% intramedullary).
The most common primary tumors that metastasize to the epidural spinal compartment are listed below:
• Lymphoma (usually due to systemic spread)
• Bronchial carcinoma
• Breast carcinoma
• Prostatic carcinoma
• Gastrointestinal tract neoplasms
• Melanoma
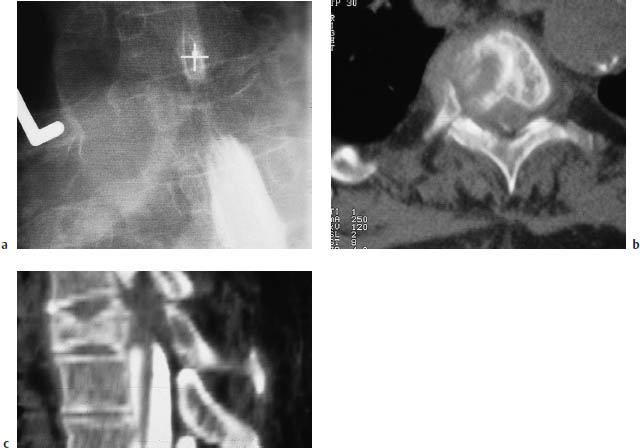
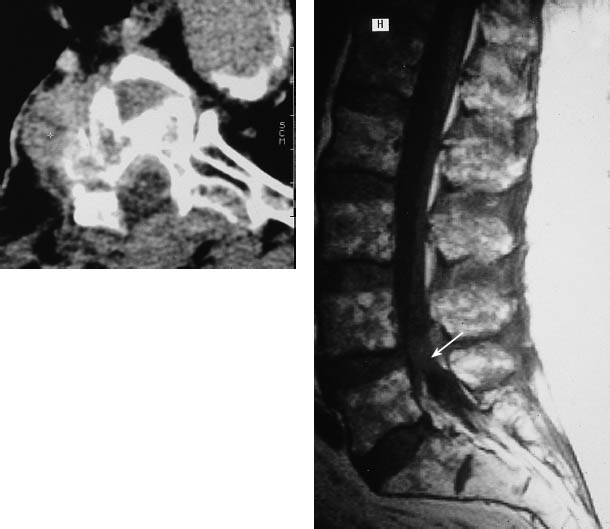
Fig. 13.3a–e Spinal metastases from bronchial carcinoma in a patient with clinical manifestations of a partial cord lesion at the T6–7 level.
a Myelography reveals a compression fracture of the T4 vertebral body, with dural sac compression and a partial block of the contrast column.
b A sagittal reformatted image of postmyelographic CT scans clearly demonstrates spinal canal encroachment by the T4 fracture, with soft-tissue components contributing to compression of the dural sac.
c The bone-window view of the affected vertebral body (postmyelographic CT) shows an extensive mass with an intraspinal component and associated bone destruction.
e A sagittal T2-weighted magnetic resonance image (MRI) of the lumbar spine in the same patient illustrates the higher sensitivity of MRI for detecting vertebral body metastases without an associated pathologic fracture. An additional intraspinal metastasis is also seen (arrow).
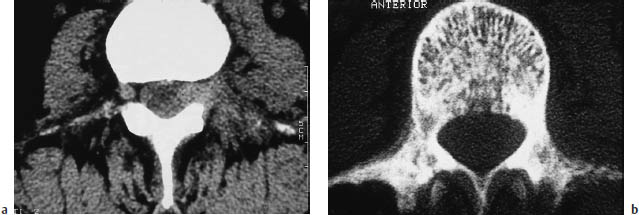
Fig. 13.4a, b Spinal metastases from breast cancer.
a CT scan with a soft-tissue window in a woman with known breast cancer and a radicular compression syndrome of the left L3 nerve root. An intraspinal-extradural metastasis was found at surgery.
b The bone-window view in the same patient shows diffuse, patchy sclerosis of the vertebral bodies reflecting diffuse involvement by osteoplastic metastases. Therapy-induced sclerosis should also be considered in breast cancer patients, however.
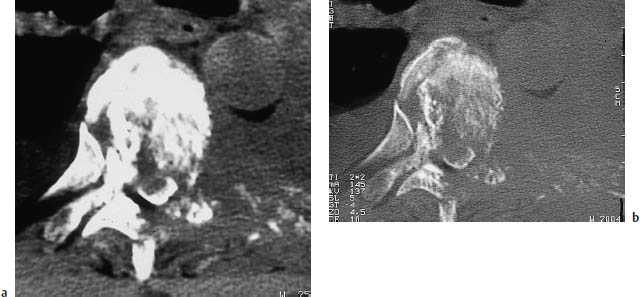
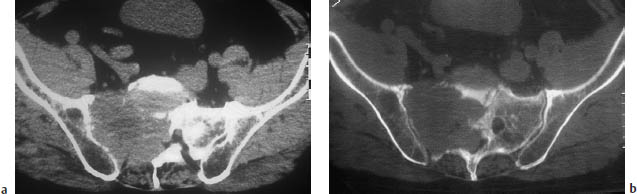
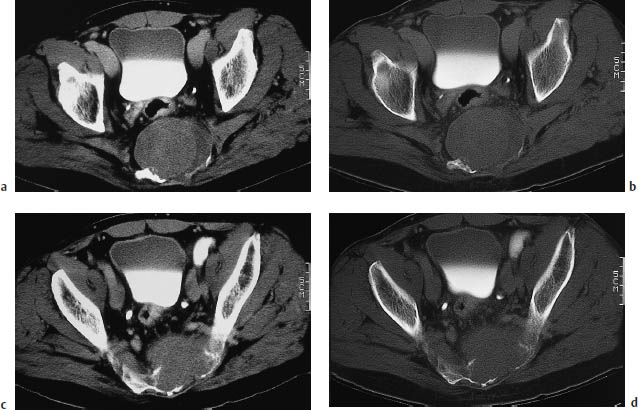
Fig. 13.6a, b A large metastatic tumor in the right lateral mass of the sacrum. The tumor has caused compression symptoms involving multiple sacral nerve roots.
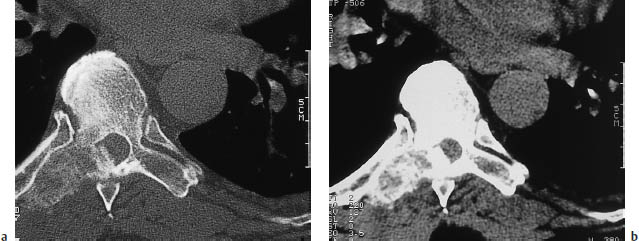
Fig. 13.7a, b A metastatic tumor in the transverse process of a thoracic vertebra.
The most common mode of spread is by hematogenous metastasis via the segmental arteries or epidural venous plexus (Batson’s plexus, Fig. 13.9).
The distribution of metastatic lesions in the cervical, thoracic, and lumbar regions is roughly proportional to the anatomic length of region. Thoracic metastases are the most common, therefore, accounting for 50–60% of lesions.
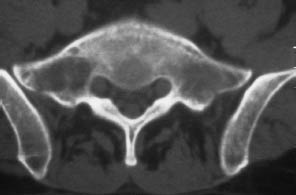
Fig. 13.8 Metastasis in the right lateral mass of the sacrum. So far, the tumor has caused only spinal cord displacement and trabecular destruction. The cortical bone is still intact.
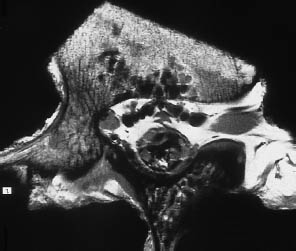
Fig. 13.9 Communication of veins. This high-resolution magnetic resonance image of a lumbar vertebra preparation clearly demonstrates the prominent epidural veins and the vertebral body veins that anastomose with them.
 Frequency
Frequency
Metastases are the most common extradural spinal masses, occurring in approximately 10% of cancer patients. Some 5–10% of malignant tumors present initially with clinical symptoms due to cord compression.
 Clinical Manifestations
Clinical Manifestations
Pain is the initial symptom in 95% of cases. It may be focal or radicular in nature, and is generally aggravated by movement or acts that raise the intra-abdominal and epidural pressures (coughing, sneezing, bearing down).
As the mass effect of the lesion increases, it can produce a variety of cord or conus compression symptoms ranging to complete cord paralysis. Progression may be insidious as the lesion grows and exerts more pressure on the cord, or symptoms may occur acutely due, for example, to progressive vertebral body destruction that culminates in a pathologic fracture.
The most serious potential complications of spinal metastases are instability of the spinal column caused by the destruction of one or more vertebral bodies and cord compression by a space-occupying lesion within the spinal canal.
 CT Morphology
CT Morphology
Multifocal occurrence is common, and most metastases enhance after intravenous contrast administration. Vertebral body destruction most frequently involves the pedicles. Osteoplastic lesions that are hyperdense on CT scans are typical of metastases from prostatic and breast carcinoma. They are particularly common after treatment initiation in breast cancer patients. Dense vertebral sclerosis (ivory vertebra) may develop in Hodgkin disease (Fig. 13.10). The treatment of bony metastases with bisphosphonates such as pamidronate (Aredia) can induce a generalized hyperdensity throughout the skeleton.
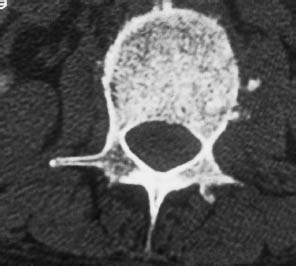
Fig. 13.10 Hodgkin disease. Bone-window CT scan of an ivory vertebra in Hodgkin disease.
Lymphoma (Figs. 13.11–13.14)
Frequency: 0.1–10% of patients with non-Hodgkin lymphoma, depending on the source.
Suggestive morphologic findings: highly variable. All three compartments may be involved, including the bone. Contiguous invasion by paraspinal tumor is common. Some tumors are hyperdense on plain CT scans and enhance after intravenous contrast administration.
Procedure: plain and postcontrast scans, including bone window.
Other studies: MRI can demonstrate the entire spinal column and is the most sensitive study for detecting medullary involvement (Fig. 13.13).
Checklist for scan interpretation:
 Craniocaudal extent?
Craniocaudal extent?
 Degree of cord compression?
Degree of cord compression?
 Bone destruction or impending instability?
Bone destruction or impending instability?
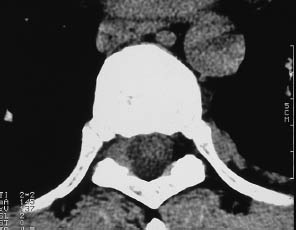
Fig. 13.11 Epidural involvement of the thoracic spine by non-Hodgkin lymphoma. There is material of soft-tissue density filling the epidural space to the right of the dural sac, displacing the epidural fat in that area.
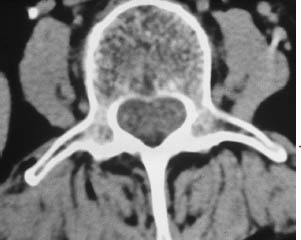
Fig. 13.12 Intradural spinal involvement by non-Hodgkin lymphoma. This unenhanced CT scan of the lumbar spine is difficult to interpret. When the diagnosis is known, however, it can be seen that the intradural space, which normally contains only cerebrospinal fluid and cauda equina fibers at this level, shows an almost uniform hyperdensity.
 Pathogenesis
Pathogenesis
Like metastases from a solid primary tumor, lymphoma can occur in all spinal compartments. Spinal involvement may be primary or metastatic. Lymphoma is sometimes difficult to distinguish from other circumscribed masses or diffusely infiltrating processes, depending on the affected compartment. The most common pattern is infiltration of the bone and invasion of the spinal canal through the intervertebral foramina by a retroperitoneal or paravertebral lesion. After entering the spinal canal, lymphoma can lead to cord ischemia through vascular compression or may compress the cord directly. Cord compression can also result from vertebral body destruction by lymphoma. Involvement of the vertebral bodies is often associated with extensive bone destruction and sclerotic changes.
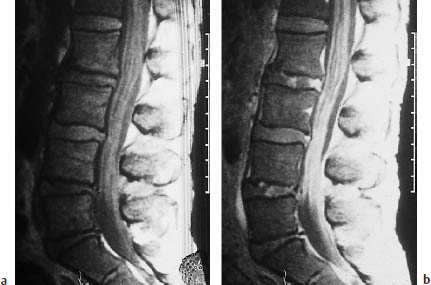
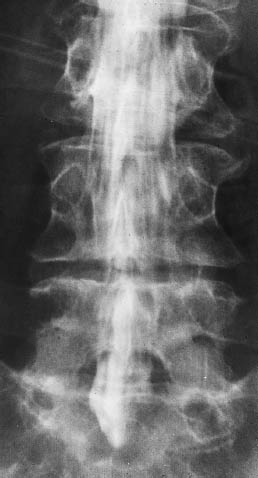
Fig. 13.14 Diffuse subarachnoid spread of lymphoma. Lumbar myelography (same patient as in Fig. 13.13) also shows diffuse lymphomatous involvement of the subarachnoid space.
Other patterns of lymphomatous involvement are:
• Leptomeningeal spread from a primary CNS lymphoma (usually diffuse, rarely focal)
• Primary intramedullary lymphoma
Primary intramedullary lymphoma is extremely rare, however, and only a few cases have been described in the world literature.
 Frequency
Frequency
Spinal involvement is present in 0.1–10% of patients with non-Hodgkin lymphoma, depending on the source. As the population infected with human immunodeficiency virus (HIV) and immunosuppressed transplant recipients have become more numerous in the past 10–15 years, there has been a marked rise in the frequency of CNS involvement by lymphoma and especially of primary CNS lymphomas. At the same time, the peak age incidence has declined from 40–70 years to the current 30–40 years.
 Clinical Manifestations
Clinical Manifestations
Clinical manifestations depend on the degree of cord compression and the effects of vascular compression. A complete cord lesion may develop in severe cases.
As noted earlier, lymphoma is often difficult to distinguish from other diseases, depending on its location and pattern of spread. Purely osseous involvement can resemble metastases from other tumors, but may feature sclerotic areas in addition to extensive bone destruction. Especially in Hodgkin disease, dense sclerosis already visible on plain films can lead to the development of an “ivory vertebra” (Fig. 13.10).
A lymphoma that has invaded the spinal canal from the retroperitoneum has a less ambiguous CT appearance, and is usually accompanied by signs of retroperitoneal lymphadenopathy.
Leptomeningeal spread typically appears as a diffuse sheet-like area or disseminated micronodular region of contrast enhancement.
Primary intramedullary lymphoma is extremely rare and is virtually indistinguishable from glioma. The fact that primary CNS lymphomas are often hyperdense on plain CT scans owing to their high cellular density is less helpful in the spine than in the cranium, due to the narrow confines of the spinal canal, as well as partial-volume and beam-hardening artifacts.
Lymphomas in all locations usually show intense contrast enhancement, especially on delayed images.
Osteogenic Extradural Masses
Chordoma (Figs. 13.15, 13.16)
Frequency: rare.
Suggestive morphologic findings: destruction of one or more vertebral bodies or the sacrum, associated soft-tissue mass, contrast enhancement.
Procedure: thin postcontrast CT slices, bone-window views.
Other studies: MRI or radionuclide bone scanning can exclude other lesions and differentiate chordoma from metastases. Compare with conventional radiographs.
Checklist for scan interpretation:
 Tumor extent and segmental location?
Tumor extent and segmental location?
 Instability?
Instability?
 Cord compression?
Cord compression?
 Pathogenesis
Pathogenesis
Chordomas are rare tumors that arise from remnants of the embryonic notochord (develops in week 4–7). Normally, the notochord differentiates into the nucleus pulposus of the intervertebral disk.
If a chordoma develops, it is usually located at one end of the notochord. This can result in a clivus chordoma, cervical chordoma, or sacrococcygeal chordoma.
There is still disagreement regarding the biologic behavior of chordomas. While many authors note that the tumor is locally invasive and causes bone destruction, they still characterize it as benign. The tumor has a high recurrence rate after excision. Distant metastases to the lung, bone, liver, and lymph nodes have also been described.
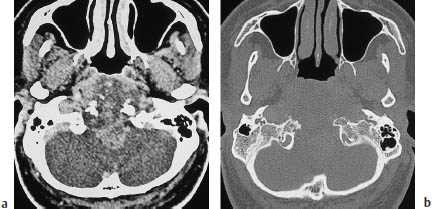
Fig. 13.15 Clivus chordoma. The tumor has completely destroyed the clivus, and is causing an extensive mass effect.
 Frequency
Frequency
Chordomas are relatively rare. They make up approximately 1–4% of “malignant” bone tumors, and their incidence is one in 2 million. While spheno-occipital chordomas affect both sexes equally, sacrococcygeal and vertebral chordomas are about twice as common in men than women. The peak age incidence is in the fifth decade.
Vertebral chordomas make up 15–20% of chordomas. The most common site of occurrence is the cervical spine, followed by the lumbar spine.
 Clinical Manifestations
Clinical Manifestations
Pain is a common but nonspecific symptom. It may be local or may radiate in a radicular distribution. Compression of the spinal cord, conus medullaris, or cauda equina frequently develops over time. Even large sacrococcygeal chordomas may cause few symptoms, however.
 CT Morphology
CT Morphology
CT often shows extensive destruction of one or more vertebral bodies or portions of the sacrum (Fig. 13.16). The destruction extends beyond the disk space, and is often associated with an adjacent soft-tissue mass, which may be quite large. An intraspinal tumor component can lead to cord or nerve root compression.
The tumor often appears hyperdense on unenhanced CT scans, which show complete destruction of the trabecular structure of the affected vertebral bodies. Most chordomas enhance after intravenous contrast administration.
Frequency: rare; almost never seen after age 30.
Suggestive morphologic findings: nonsclerotic osteolytic area with sharply defined margins and a hyperdense center (sequestrum); little or no soft-tissue or periosteal reaction; vertebral body collapse (vertebra plana).
Procedure: thin CT slices, bone window views.
Other studies: obtain conventional radiographs of other skeletal regions, especially the skull, to check for multiple lesions.
Checklist for scan interpretation:
 Location and extent of the lesion?
Location and extent of the lesion?
 Signs of instability?
Signs of instability?
 Pathogenesis
Pathogenesis
Eosinophilic granuloma is one of a group of granulomatous disorders (Abt-Letterer-Siwe disease, Hand-Schüller-Christian disease) that are known collectively as histiocytosis X. Eosinophilic granuloma is the mildest form.
Histopathologically, eosinophilic granuloma involves a local infiltration of the bone with mononuclear and eosinophilic granulocytes that form a soft-tissue mass. As the disease progresses, the mass causes lysis of the bone.
Some tumors show a propensity for spontaneous remission.
One treatment option is resection of solitary lesions. Multiple lesions are better managed by chemotherapy or low-dose radiotherapy if the affected vertebral bodies show signs of impending instability.
 Frequency
Frequency
Eosinophilic granuloma is the most common and mildest variant of histiocytosis X. It affects children and young adults almost exclusively. A useful rule of thumb is that eosinophilic granuloma can be eliminated as a differential diagnosis in patients over 30 years of age. The peak age incidence is approximately 5–10 years, and males predominate by a ratio of about 3:2. Involvement is monostotic in 50–75% of patients. The spine is the third most common site of occurrence after the calvarium and mandible, accounting for 20–25% of cases.
 Clinical Manifestations
Clinical Manifestations
The cardinal symptom in most cases is local pain.
 CT Morphology
CT Morphology
Eosinophilic granuloma appears as an osteolytic lesion on CT scans, often causing a dramatic decrease in vertebral body height. Generally the vertebral arches are spared. As it continues to lose height, the vertebral body assumes the shape of a thin disk (vertebra plana), which is typically seen in the thoracic region. Often, there is a central hyperdensity caused by the sequestration of bone material within the soft-tissue mass. The margins of the osteolytic lesion are well defined, but are not sclerotic. Usually, there is little or no evidence of an associated soft-tissue or periosteal reaction.
Giant-Cell Tumor (Osteoclastoma)
Frequency: rare; the spine is the primary location in only 5% of cases.
Suggestive morphologic findings: expansile, destructive mass with associated cortical destruction and nonsclerotic margins. Shows nonhomogeneous enhancement after contrast administration.
Procedure: thin CT slices, intravenous contrast administration, bone-window views.
Other studies: conventional radiographs, radionuclide bone scan.
Checklist for scan interpretation:
 Location and extent (may be marked on the skin if desired).
Location and extent (may be marked on the skin if desired).
 Associated intraspinal soft-tissue mass?
Associated intraspinal soft-tissue mass?
 Instability or risk of fracture?
Instability or risk of fracture?
 Pathogenesis
Pathogenesis
Giant-cell tumor is a locally aggressive primary bone tumor that arises from osteoclasts (synonym: osteoclastoma). Spinal involvement is described in about 5% of cases. Approximately 15% of giant-cell tumors are classified as malignant, but the radiologic, clinical, and even histologic findings may be insufficient for confident benign-malignant differentiation. Both benign and malignant forms have a high recurrence rate, even after radical excision.
The tumor most commonly affects women 20–30 years of age.
Morphologically, giant-cell tumor is an osteolytic lesion associated with a reddishgray soft-tissue mass that may contain cysts, hemorrhagic areas, and necrosis. The cortex is usually thinned from the inside, and the zone of bone destruction does not develop sclerotic margins. A periosteal reaction may be present.
An important differentiating criterion is that giant-cell tumors always arise from epiphyseal plates that have already closed. This means that they occur only after the cessation of skeletal growth.
The most common sites of occurrence of giant-cell tumors are the epiphyses of the long tubular bones. In the spinal column, the sacrum is commonly affected. Generally the vertebral body is involved first, with subsequent extension to the vertebral arches. Associated soft-tissue masses have been described.
Treatment is geared toward the histologic tumor grade, and ranges from meticulous curettage of a benign giant-cell tumor to radical excision of a malignant form.
 Frequency
Frequency
Giant-cell tumor is a rare neoplasm, and only about 5% affect the spinal column primarily. The peak age of occurrence is between 20 and 40 years, and women are affected about twice as often as men.
 Clinical Manifestations
Clinical Manifestations
Common nonspecific symptoms are local pain, limitation of motion, and occasional pathologic compression fractures. An intraspinal or paravertebral soft-tissue mass may cause symptoms of cord or nerve root compression as it enlarges.
 CT Morphology
CT Morphology
Giant-cell tumor appears on CT as an expansile, destructive tumor that is frequently associated with cortical destruction. Generally there is no detectable sclerotic margin. An associated soft-tissue mass may be visible. The tumor usually shows nonhomogeneous enhancement after contrast administration. It cannot be classified as benign or malignant based on its imaging appearance alone.
Osteoid Osteoma, Osteoblastoma
Frequency: relatively common, accounting for approximately 40% of benign spinal tumors and 1.4% of spinal tumors.
Suggestive morphologic findings: expansile tumor with a central, very vascular enhancing nidus and sclerotic margins.
Procedure: thin CT slices, intravenous contrast administration, bone-window views.
Other studies: the central nidus is often visible on plain radiographs. Bone scans show marked radiotracer uptake.
Checklist for scan interpretation:
 Location (may be marked on the skin if desired).
Location (may be marked on the skin if desired).
 Instability?
Instability?
 Impending cord or root compression?
Impending cord or root compression?
 Additional studies to exclude multiple lesions (e.g., radionuclide scan).
Additional studies to exclude multiple lesions (e.g., radionuclide scan).
 Pathogenesis
Pathogenesis
Osteoid osteoma is a benign tumor that arises from osteoblasts. The classic radiographic appearance is that of a small, hyperdense tumor with a central lucency called the nidus. Only the nidus presents the histologic features of osteoid osteoma: a rounded focus in cancellous bone surrounded by a sclerotic zone of cortical thickening.
The tumor is composed of numerous osteoid trabeculae, mostly uncalcified, surrounded by osteoblasts and osteoclasts in a capillary-rich stroma.
Osteoblastoma differs histologically from osteoid osteoma mainly by its richer vascular supply. Generally, a tumor that is 15–20 mm or larger is classified as an osteoblastoma. Because of its size, osteoblastoma is particularly likely to cause cord or nerve root compression.
In one study, 22% of patients with spinal osteoid osteomas had neurologic deficits while 28% of patients with spinal osteoblastomas showed signs of myelopathy.
With both forms, adolescents are predominantly affected. Lesions in vertebral bodies are most commonly located in the vertebral arch. Although both entities are benign bone tumors, complete surgical excision is indicated because of the potentially severe bone pain, which worsens at night.
 Frequency
Frequency
Primary spinal involvement occurs with approximately 40% of osteoblastomas and 25% of osteoid osteomas. Together, these tumors make up approximately 40% of benign spinal bone tumors and 1.4% of vertebragenic tumors in general. Males predominate by a 2:1 ratio. The peak age incidence is about 20–30 years.
 Clinical Manifestations
Clinical Manifestations
The classic clinical symptom is local pain that worsens at night and is relieved by aspirin. This is not a consistent feature, however, as it occurs in only 40% of patients with spinal osteoid osteoma and only 25% of patients with osteoblastoma. The second most common symptom after pain is scoliosis, which may be painful or antalgic. The convexity is usually toward the contralateral side. Radicular pain and/or neurologic deficits also occur in 50% of patients.
 CT Morphology
CT Morphology
Osteoid osteoma and osteoblastoma typically present on CT as an expansile tumor that often affects the vertebral arches primarily. The tumor has a very vascular central nidus that is surrounded by a sclerotic zone and enhances after intravenous contrast administration.
Aneurysmal Bone Cyst
Frequency: the second most common benign spinal tumor; predominantly affects women under 30 years of age.
Suggestive morphologic findings: an expansile, predominantly cystic mass that is hypodense on plain CT scans, and has a nonhomogeneous internal structure. Shows intense, nonhomogeneous enhancement after contrast administration. May show a clam-shell pattern of periosteal calcification.
Procedure: thin CT slices before and after contrast administration (may use bolus injection), bone-window views.
Other studies: MRI can detect intracystic hemorrhage and fluid levels. Conventional radiographs.
Checklist for scan interpretation:
 Extent and location (may be marked on the skin if desired).
Extent and location (may be marked on the skin if desired).
 Intraspinal mass?
Intraspinal mass?
 Risk of fracture?
Risk of fracture?
 Pathogenesis
Pathogenesis
Aneurysmal bone cyst is a benign osteolytic bone lesion. Unlike a juvenile bone cyst, however, it is not a primary lesion but develops in response to previous bone injury. It may also accompany a giant-cell tumor or fibrous dysplasia. Some authors disagree with this view of the pathogenesis—noting, for example, that it does not explain why 90% or more of patients are younger than age 30.
Aneurysmal bone cysts occur most frequently in the spine (approximately 30%), the long bones, and the pelvis. They usually consist of an eccentric osteolytic lesion, often expansile, bordered by a hernia-like outpouching of periosteum that breaks through the cortex and extends along adjacent vertebra, forming a beehive-like expansion that mimics the internal involvement of multiple vertebrae. The posterior elements are more commonly affected.
 Frequency
Frequency
Aneurysmal bone cyst is the second most common benign spinal tumor. Approximately 20–30% of aneurysmal bone cysts occur in the spine. Sites of predilection are the posterior vertebral elements of the lower thoracic and upper lumbar spine. Most patients are under age 30. Females from age 10–20 are most commonly affected.
 Clinical Manifestations
Clinical Manifestations
Most patients have a painful swelling or merely local hyperesthesia or hyperalgesia. Up to 5% of patients sustain a pathologic fracture.
 CT Morphology
CT Morphology
Aneurysmal bone cyst typically appears as a hypodense, expansile, cyst-like mass with a nonhomogeneous internal structure. It shows intense, nonhomogeneous enhancement after contrast administration, especially when a bolus injection is used (Fig. 13.17).
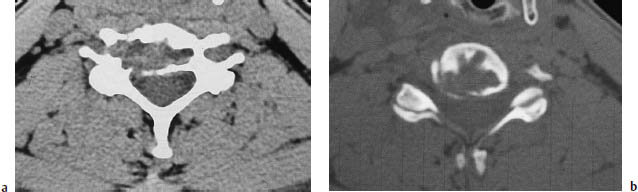
Fig. 13.17a, b Aneurysmal bone cyst. CT typically shows a large, expansile, osteolytic intraosseous mass without sclerotic margins. This lesion has penetrated the cortex, but has not yet formed an intraspinal mass. The postcontrast scan usually shows an intense, nonhomogeneous pattern of enhancement.
Vertebral Hemangioma (Figs. 13.18, 13.19)
Frequency: the most common benign spinal tumor, present in approximately 9–12% of the general population. A common incidental finding.
Suggestive morphologic findings: punctate accentuation of hypertrophic trabeculae in an axial CT scan. May or may not enhance after contrast administration.
Procedure: thin CT slices, bone-window views.
Other studies: vertebral hemangioma is visible on conventional radiographs if it involves at least one-third of the vertebral body. On MRI, hemangiomas with lipomatous changes are hyperintense on T1- and T2-weighted images; expansile hemangiomas are isointense on T1-weighted images and hyperintense on T2-weighted images. Radionuclide scan can detect existing compression fractures.
Checklist for scan interpretation:
 Does CT confirm or exclude a suspected vertebral hemangioma?
Does CT confirm or exclude a suspected vertebral hemangioma?
 Compression fracture or risk of fracture?
Compression fracture or risk of fracture?
 Expansile mass causing cord or root compression?
Expansile mass causing cord or root compression?
 Pathogenesis
Pathogenesis
Cavernous hemangiomas are benign bone lesions. They are often multifocal, and become more numerous with aging. In approximately one-third of cases, up to five noncontiguous levels are affected. The most common sites of occurrence are the lower thoracic and upper lumbar spine. Hemangiomas are extremely rare in the cervical vertebrae.
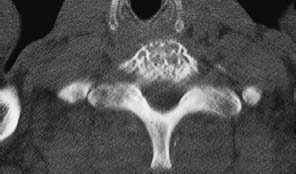
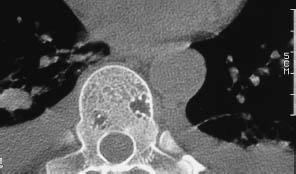
Fig. 13.19 Thoracic vertebral hemangioma. While some hemangiomas involve the entire vertebral body, there are circumscribed forms that are not usually seen on conventional radiographs, like these two small hemangiomas in a thoracic vertebra.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



