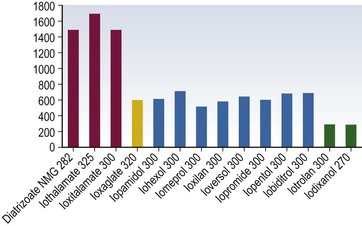
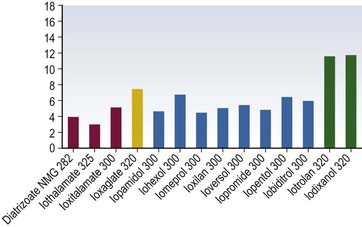
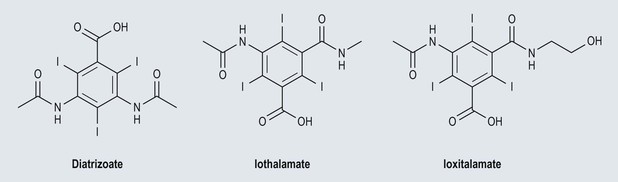
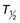Henrik S. Thomsen, Peter Reimer Contrast media are commonly used for imaging to enhance the differences of structures or fluids within the body tissue. They may be used in radiological procedures such as radiography, fluoroscopy, angiography, computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound. Various types of contrast media exist for each technique, and their applications depend on the chemical and physical properties of the agents. The ideal contrast medium does not exist. It should be possible to inject the ideal agent fast or drink it. It should leave the body as soon as possible—after it has enhanced the structures in question—in unchanged form and without causing any harm to the body, including adverse reactions. Almost all agents cause discomfort and adverse reactions to some degree. Discomfort, e.g. metal taste and/or feeling of warmth, is frequent. Serious reactions requiring treatment are infrequent. Acute non-renal adverse reactions are the same for all types of contrast media, whereas there are differences regarding acute renal adverse reactions, late reactions and very late reactions. Most of the agents are out of the body within 24 hours if the patient has normal renal function. It may take weeks before the agent is out of the body if the patient has severely reduced renal function. Iron-based agents enter the natural circulation like any other iron ion. Hepatobiliary gadolinium-based agents are partially excreted via the hepatobiliary system, whereas the ion manganese is excreted solely by the liver. Barium products or iodine-based oral agents stay in the intact gastrointestinal tract. Products like air, carbon dioxide, tap water, and juices containing manganese, e.g. blueberry juice, pineapple juice, can be used as contrast media, but their use is beyond the scope of this chapter. A positive contrast medium attenuates the X-rays significantly stronger than the body’s soft tissues and in the appropriate concentration and dose could be applied in—or even reach—the body system which is imaged. They can be divided into (1) water-soluble (iodine-containing) substances and (2) non-water-soluble (barium) compounds. The atoms barium and iodine absorb X-rays significantly in a wavelength of 0.02 to 0.3 nm equal to that used in diagnostic radiology. A negative contrast agent absorbs X-rays less than the soft tissue does. Water and air can be used as negative contrast agents. The gastrointestinal tract is most frequently studied with very poorly soluble barium sulphate, BaSO4, which is administered orally or rectally, in a finely divided aqueous suspension with 0.3 to 1 g dry weight per millilitre. Barium sulphate is normally not absorbed during passage through the digestive tract. It may overflow into the lungs and leak into the mediastinum, the tissue around the rectum or intraperitoneal cavity causing granuloma and occasional fatal reactions.1 Therefore, when one suspects aspiration, a fistula between the oesophagus and lungs, or perforation of the gastrointestinal tract, the use of barium sulphate should be avoided. Leakage into the vasculature is life threatening and it is therefore important to be aware of this during the study, so treatment can be initiated immediately. Side effects include barium causing or exacerbating constipation, worsening ulcerative colitis inflammation, e.g. peritonitis through perforation (Table 2-1). Iodine (atomic number 53 and atomic weight of 127) is the only element that is proved satisfactory for general use as an intravascular contrast medium for radiography including angiography and CT.2 The iodine provides the radiopacity; the other elements of the contrast medium molecule provide no radiopacity but act as carriers of the iodine, greatly increasing the solubility and markedly reducing the toxicity of the molecule.3 The problem has always been how to pack the iodine so it may be delivered safely into very sensitive arterial systems (e.g. brain, heart, kidneys) in the very large amounts required to produce adequate radiopacity.4 Some agents can even be administered into the cerebrospinal fluid without causing major problems. Organic carriers of iodine will probably remain the basis of all intravascular contrast media for the foreseeable future. Since the 1950s, four chemical varieties of iodine-based contrast media in clinical use have been introduced.5 All four are tri-iodo benzene ring derivatives with three atoms of iodine at 2,4,6 positions in monomers and six atoms of iodine per molecule of the ring atom in dimers; they are very hydrophilic; have low lipid solubility, low toxicity, low binding affinities for protein, receptors or membranes; and have molecular weights less than 2000.4 Their osmolality and viscosity appear in Figs. 2-1 and 2-2, respectively. All ionic monomers are salts with sodium or meglumine (N-methylglucamine) as the non-radiopaque cation and a radiopaque tri-iodinated fully substituted benzoic acid ring as the anion. These anions include diatrizoate, ioxitalamate and iothalamate. Each molecule completely dissociates in water into two ions—one non-radiopaque cation and one tri-iodinated radiopaque anion, giving iodine a particle ratio of 3 : 2. They are very hypertonic ~1600 mosmol/kg water at 300 mgI/kg compared with the physiological osmolality of 300 mosmol/kg water (Fig. 2-1). High-osmolar monomeric contrast media are rarely used intravascularly nowadays and have almost been replaced by non-ionic low-osmolar contrast media. Ioxaglate is the only compound in this group. It is a mixture of sodium and meglumine salts of a mono acidic double benzene ring with each benzene ring having three atoms of iodine at C2, C4, C6 positions. The total molecule, therefore, contains six atoms of iodine and in solution each molecule dissociates into one radiopaque hexa-iodinated anion and one non-radiopaque cation (sodium and/or meglumine). Ioxaglate, therefore, has an iodine-to-particle ratio of 6 : 2 or 3 : 1. The osmolality is similar to that of the non-ionic monomers. The first agent was metrizamide introduced by Torsten Almén in 1969.2 Today, second-generation non-ionic monomers (iohexol, iopamidol, iopromide, ioversol, ioxilan, iomeron, iobitridol, iopentol, iobiditrol) have taken over and are much more stable, much more soluble and much less toxic. None of these molecules dissociate in solution. They are iodinated non-ionic compounds and therefore in solution they provide three atoms of iodine to one osmotically active particle (the entire molecule), producing an iodine-to-particle ratio of 3 : 1. They have less than half of the osmolality of HOCM. They have an osmolality of about 600 mosmol/kg water at a concentration of 300 mgI/mL compared with the physiological osmolality around 300 mosmol/kg (Fig. 2-1). Isomenol, iotrolan, iodixanol and GE-145 are examples of non-ionic chemicals. They do not dissociate in solution. Each molecule, therefore, provides in solution six atoms of iodine for one molecule, i.e. an iodine-to-particle ratio of 6 : 1. Non-ionic dimers are sold as physiologically isotonic (300 mg/kg water) in solution at 300 mgI/mL. Various electrolytes (i.e. trometamol, sodium chloride, calcium chloride dihydrate, sodium calcium edetate) are added to keep the medium at isotonic levels at all iodine concentrations. Having an osmolality lower than that for the non-ionic monomers has a price: the viscosity is high even at body temperature (Fig. 2-2). The high viscosity makes it more difficult to inject the agent through thin IV lines or angiography catheters with small lumen. Therefore, the contrast agents are typically preheated before injection. None of the four chemical types of contrast media have marked pharmacological actions. This is highly desirable as they are used purely for imaging and not as therapeutic drugs. After intravascular injection, all of the four types are distributed rapidly because of high capillary permeability into the extravascular, extracellular space (except in the central nervous system as they do not pass an intact blood–brain barrier). They do not enter the interior of blood cells or tissue cells. Protein binding is minimal. The agents follow two-compartment kinetics (Fig. 2-7). Contrast substances are excreted unchanged by glomerular filtration. In people with normal glomerular filtration rate (GFR; >60 mL/min/1.73 m2) more than 80% is excreted after 4 hours, and 98% after 24 hours. In renal disease, it can take days/weeks depending on their impairment. In most patients, small amounts (<1%) are excreted via the bile, but in some patients a little more biliary excretion is seen; on the following day, contrast medium can be found in the gallbladder at CT. This is normal. The hydrophilic contrast agents are not absorbed from the intact gastrointestinal tract and, in particular, the high-osmolar contrast agents will draw water into the gastrointestinal lumen, with the risk of dehydrating the patient. Very large amounts of iodine used to be necessary because of the low sensitivity inherent in conventional film-screen radiography. This low sensitivity to iodine is not an inherent property of the X-ray beam but is due to the low sensitivity of projection images where everything between the X-ray tube and the detector is added up to a single image. Techniques used in CT imaging and digital subtraction angiography (DSA) are more sensitive to minor differences in iodine concentration. In complex cardiac interventional cases, the use of more than 1000 mL of iodine-based contrast medium has been reported,6 indicating the relative safety of the agents even when used in large amounts. However, one should never use more contrast medium than necessary to confirm the presence of a lesion or to confirm the absence of a lesion in any patient. MR contrast agents are diagnostic pharmaceutical compounds, which mainly contain paramagnetic metal ions that affect MR signal properties of surrounding tissues. Paramagnetic agents are positive enhancers that reduce the T1 and T2 relaxation times and increase tissue signal intensity on T1-weighted MR images and have nearly no effect on T2-weighted images (Fig. 2-8). Superparamagnetic ions have a signal-enhancing effect on T1-weighted images at low concentrations, whereas in higher concentrations they have a negative effect on the signal from the surrounding tissue on T2/T2*-weighted images and nearly no effect on T1-weighted images. Copper (Cu2+), manganese (Mn2+) and gadolinium (Gd3+) were considered as potential paramagnetic ions to be used for MRI in the 1980s. However, Gd (atomic number 64 and atomic weight of 157) turned out to be the most powerful, with seven unpaired electrons, but, unfortunately, the most toxic of those ions.7 Therefore, it is necessary to encapsulate it by a chelate. As a supraparamagnetic ion, iron (Fe2+) was introduced. The first MR contrast agent to be introduced into clinical practice was the gadolinium diethylenetriamine pentaacetic acid salt (Gd-DTPA).8 The chelate DTPA was already known from its use in nuclear medicine (99mTc-DTPA). Several gadolinium-based MR contrast agents are currently available for clinical use in addition to Gd-DTPA (Fig. 2-9). Over the years, contrast media based on manganese and iron for intravenous use have been introduced, but most of them have been taken off the market for various reasons (production problems, limited sale, etc.). Two principally different chelates were introduced to detoxify gadolinium: (1) linear or acyclic and (2) macrocyclic or cyclic.5 Just like the iodine-based contrast media, both are available as ionic and non-ionic.5 Physical data9 for the various gadolinium-based agents are shown in Table 2-2 and Figs. 2-10 and 2-11. Three of the nine agents have more or less affinity to albumin (4–90%). The differences in osmolality (Fig. 2-10) do not have any effect on the incidence of adverse reactions compared with the situation for iodine-based contrast agents. The lack of a difference is likely due to the small amounts of the agent used for MRI.10 The viscosity of the agents is low and unimportant. Gadolinium in itself does not change the signal; it is the surroundings that change signal intensity when the agent is there.11 The effect increases when gadolinium is bound to a chelate and the addition of albumin increases the effect further.9 Relaxivity of the various agents at 1.5 and 3 T are shown in Fig. 2-11. The increase in field strength has no major impact on the relaxivity of the agents except for the gadofosveset, which is highly bound to albumin. The molecular structure of the chelate affects the stability of the molecules, i.e. how tightly the gadolinium is held within them.12,13 In vitro measurements of the chemical stability of gadolinium-based contrast media show that the cyclic chelates are the most stable and that the non-ionic linear chelates are the least stable.14 The conditional stability constant and thermodynamic stability constant are shown in Table 2-2. In the cyclic molecules, the gadolinium is caged in the molecular ring (Fig. 2-6), while in the acyclic molecules the gadolinium is held less strongly. Ionic acyclic molecules are generally more stable than non-ionic. In vivo animal measurements support these findings, with three times more gadolinium retention in the tissues of rats and mice with normal renal function two weeks after the non-ionic acyclic agent gadodiamide than after the ionic acyclic agent gadopentetate dimeglumine.13 Only very small amounts of gadolinium were retained in the tissues after the cyclic agents gadobutrol, gadoterate meglumine and gadoteridol were used.15 The pharmacokinetics of all extracellular MR contrast agents are similar to that of iodine-based, water-soluble contrast media. The agents have low molecular mass and they rapidly diffuse into the interstitial extravascular space after intravenous injection. Reverse diffusion into the intravascular compartment also occurs and a state of equilibrium between diffusion in and out is usually reached within two hours. Gadolinium chelates are constantly eliminated from the intravascular compartment by passive glomerular filtration. Subsequently, more than 95% of the injected dose is excreted in urine within 24 hours in the presence of normal renal function. In patients with severely reduced renal function, it may take several days/weeks before the agents have left the body. During the prolonged elimination, transmetallation may occur, particular in acyclic non-ionic agents.13 Gadolinium is liberated from the chelate and another ion, e.g. zinc and iron, has taken its place. Very small amounts <0.1% are eliminated via feces. Extracellular MR contrast agents do not cross-intact specialised vascular blood–brain barrier nor bind to proteins or receptors and there is no intracellular penetration. These agents accumulate in tissues with abnormal vascularity (malignant and inflammatory lesions) and in regions where the blood–brain barrier is disrupted. Because of their rapid equilibration in the interstitial space of both normal and tumours/inflammatory tissues, use of dynamic MRI after bolus injection utilising the narrow imaging window with a transiently increased tumour/inflammatory to normal tissue contrast is recommended. Gadobenate dimeglumine has a capacity for weak and transient protein binding and is eliminated through both the renal and hepatobiliary pathways.16 The hepatic uptake represents 2–4% of the injected dose. Otherwise, it behaves as a conventional extracellular contrast agent in the first minutes following intravenous administration and as a liver-specific agent in a later delayed phase (40–120 min after administration) when it is taken up specifically by normal functioning hepatocytes. Gadofosveset trisodium is a blood pool agent with high affinity to albumin.16 The biological half-life is 12 times longer than that for extracellular agents in patients with normal renal function (18 hours). Gadoxetic acid is a hepatobiliary-specific, gadolinium-based contrast agent with 10% protein binding and 50% excretion by the hepatocytes, providing clinically useful signal characteristics in the hepatobiliary phase.16 Generally, the recommended dose for clinical use of the extracellular agents is 0.1 mmol/kg but up to 0.3 mmol/kg may be used, particularly in MR angiography. Because of the protein binding, three organ-specific agents can be given in doses lower than those of the extracellular agents and similar diagnostic results can be obtained. In no case should one ever use more contrast medium than necessary to verify a lesion or rule out its presence. These comprise superparamagnetic iron oxide (SPIO) that is administered intravenously or orally.17 The iron is covered by dextran or carbodextran and is produced in three sizes: large, medium and ultra small. The medium-sized particles accumulate within the Kupffer cells in the normal liver within less than 60 minutes, resulting in reduction in signal intensity of the liver at T2*-weighted gradient-echo imaging. Lesions containing Kupffer cells demonstrate signal reduction, whereas lesions that are Kupffer cell-depleted retain high signal. Some ultra small particles (USPIO) also accumulate in normal lymph nodes or in their portion with normal tissue and in other reticuloendothelial system (RES)-containing organs such as liver, spleen or bone marrow during the 24 hours after administration.18,19 The large particles are used for enhancement of the gastrointestinal tract. Currently, iron-based contrast media are no longer marketed in many countries. Manganese-based agents can be infused intravenously or be administered orally. Compared with other ions, manganese is selectively taken up by the hepatocytes and excreted via the bile (Fig. 2-12).20 The uptake is correlated with the mitochondrial function and is thus a sign of the oxygenation of the hepatocyte. After infusion, manganese can be seen in the pancreas, adrenal glands and the pituitary, but not after oral intake, which also opacifies the bowel content. Manganese is a relatively non-toxic ion, but in high doses it may be toxic; it may accumulate, for example, in the brain, where it may cause degenerative lesions with Parkinsonism as a result. Therefore, manganese may not be administered in high doses into a peripheral vein. A normal liver removes 80 to 90% of the manganese during a single passage. Manganese-based contrast agents for signal-enhanced lumen filling of the gastrointestinal tract have also been on the market. For the time being, manganese-based contrast media are no longer marketed in most countries. The principle of tissue specificity means directing a contrast agent towards a certain tissue like liver tissue or the intravascular space. The most thoroughly investigated and approved tissue-specific contrast agents relate to hepatic MRI. A variety of liver contrast agents have been developed for contrast-enhanced MRI of the liver, designed to overcome the limitations of unspecific tissue uptake by extracellular low-molecular-weight gadolinium chelates. The two main classes of liver-specific contrast agents are SPIO, with uptake via the reticuloendothelial system mainly into the liver and spleen, and hepatobiliary contrast agents, with uptake into hepatocytes followed by variable biliary excretion. Two hepatobiliary contrast agents—gadobenate dimeglumine with somewhat weak hepatic uptake and subsequent biliary excretion and gadoxetic acid with a much higher biliary uptake rate and stronger biliary excretion—are already clinically approved in many countries. Hepatobiliary agents exhibit different features for the detection and characterisation of liver tumours. Enhancement during the distribution phase of contrast agents mainly depends on tumour vascularity and its blood supply, while enhancement on delayed images is characterised by the cellular specificity of MR contrast agents. Therefore, enhancement characteristics of hepatobiliary contrast agents are applicable to the diagnosis of primary hepatocellular liver tumours.21 Blood pool contrast agents are specifically designed through persistence in the intravascular space without the passage to extravascular interstitial or extracellular fluid spaces that is the hallmark of conventional extracellular contrast (i.e. their vascular half-life is much greater than that of the extracellular agents). Furthermore, the high protein binding causes higher relaxivity, allowing the use of smaller amounts of gadolinium than of the extracellular agents. The group of ‘blood-specific’ paramagnetic intravascular contrast agents is characterised by a prolonged circulation time within the blood pool.22 The agent resembles a low-molecular-weight gadolinium complex with strong binding to albumin. The compounds demonstrated an excellent enhancement of the vascular space in clinical setting. Again, somewhat weak protein binding is also exhibited by gadobenate dimeglumine, with increased intravascular signal during contrast-enhanced MRA compared with non-specific low-molecular-weight gadolinium chelates.23 Gadolinium attenuates X-rays. Hence, their use for radiographic examinations instead of iodinated contrast media has been advocated in patients with a history of serious adverse reactions to iodinated contrast media (a gadolinium-based contrast medium is definitely safer than 4 days in an intensive care unit due to an anaphylactoid reaction to an iodine-based contrast agent) and in patients undergoing imminent thyroid treatment with radioactive iodine providing the renal function of these patients is normal. In patients with renal impairment, gadolinium-based contrast media should not be used for radiographic examinations as they can induce nephrotoxicity at the doses required to produce adequate radiographic enhancement. It is also important to point out that gadolinium-based contrast agents have not been approved for radiographic examinations or intra-arterial injection and the doses approved for MR examinations will provide only a limited enhancement in radiographic examinations. Thus, its use is definitively off-label. The development of ultrasound contrast agents, which perform as blood pool tracers, has overcome some of the limitations of conventional B-mode and colour or power Doppler ultrasound and enables the display of parenchymal microvasculature. Depending on the contrast agent and the ultrasound mode, the dynamic lesion enhancement pattern is visualised during intermittent or continuous imaging. An inherent advantage of contrast-enhanced ultrasound is the possibility of assessing the contrast enhancement patterns in real time with a substantially higher temporal resolution than other imaging techniques, without the need to predefine time points or to perform bolus-tracking. Furthermore, administration can be repeated due to the favourable safety profile and patient tolerance of ultrasound contrast agents. Ultrasound contrast media for intravenous injections are usually gas-filled microbubbles with a mean diameter less than a red blood cell (i.e. 2–6 µm). They are composed of a shell of biocompatible materials, including proteins, lipids, or biopolymers containing a filling gas. The contrast agents can be described according to the concentration of particles, size of particles or microbubbles, volume of gas, kind of gas, kind of shell, additives, etc. The microbubble shell may be stiff (e.g. denaturated albumin) or flexible (phospholipids) and has a thickness ranging from 10 to 200 nm. High-molecular-weight and low-solubility filling gases (perfluorocarbon or sulphur hexafluoride) produce a raised vapour concentration inside the microbubble relative to surrounding blood and increase the microbubble stability in the peripheral circulation.24 There are currently three principally different agents available: (1) air with a galactose and palmitic acid as a surfactant; (2) octafluoropropane (perflutren) with an albumin shell or a lipid shell; and (3) sulphur hexafluoride with a phospholipid shell. From 10 to 15 min after injection, the microbubble gas content is exhaled via the lungs, while the components of the shell are metabolised or filtered by the kidney and eliminated by the liver. In general, ultrasound contrast agents can be detected with any ultrasound technique. However, adequate detection of such small amounts of contrast agent, particularly against the background of a strong tissue signal (when used for imaging of blood circulation in the parenchyma of solid organs), requires the use of a dedicated imaging technique. Contrast-enhanced ultrasound, contrast-enhanced CT or contrast-enhanced MRI are not equivalent, as ultrasound contrast media have different pharmacokinetics and are confined to the intravascular space, whereas the majority of currently approved contrast agents for CT and MRI are rapidly cleared from the blood pool into the extravascular/extracellular space. The ultrasound contrast agents increase the ultrasound backscatter and therefore are useful in the enhancement of echogenicity for the assessment of blood flow (Fig. 2-13). While conventional ultrasound can detect high concentrations of microbubbles, in practice their assessment usually requires contrast-specific imaging modes. Contrast-specific ultrasound modes are generally based on the cancellation and/or separation of linear ultrasound signals from tissue and utilisation of the non-linear response from microbubbles. Non-linear response from microbubbles is based on two different mechanisms: (1) non-linear response from microbubble oscillations at low acoustic pressure, chosen to minimise disruption of the microbubbles, and (2) high-energy broadband non-linear response arising from microbubble disruption. Non-linear harmonic ultrasound signals may arise also in tissues themselves due to a distortion of the sound wave during its propagation through the tissue. The extent of this harmonic response from tissue at a given frequency increases with the acoustic pressure, which is proportional to the mechanical index. Low solubility gas ultrasound contrast agents are characterised by the combination of improved stability with favourable resonance behaviour at low acoustic pressure. This allows minimally disruptive contrast-specific imaging at low mechanical index and enables effective investigations over several minutes with the visualisation of the dynamic enhancement pattern in real time. Low mechanical index techniques, furthermore, lead to effective tissue signal suppression, as the non-linear response from the tissue is minimal when low acoustic pressures are used. Ultrasound with air-filled microbubbles at high pressure is dependent on microbubble disruption, which is a significant limitation for real-time imaging. Ultrasound contrast agents are generally safe with a low incidence of side effects. They are not nephrotoxic and do not interact with the thyroid gland and it is, therefore, not necessary to perform laboratory tests before their administration. The incidence of severe hypersensitivity or anaphylactic reactions is lower than with current iodine-based contrast agents and to a lesser degree with MR contrast agents. Ultrasound agents are not approved for use during pregnancy. Breastfeeding is a contraindication in some countries. Life-threatening anaphylactic reactions have been reported with a rate of less than 0.002%. Previous allergic/anaphylactic reaction to iodine- or gadolinium-based contrast agents does not necessitate any prophylaxis since the two types of agent are completely different. There is a theoretical possibility that the interaction of diagnostic ultrasound and ultrasound contrast agents could produce bioeffects. In vitro cellular effects that have been observed include sonoporation, haemolysis and cell death. Although observed in vitro, such bioeffects may have relevance for the in vivo situation, as they result from interactions between gas bodies and cells. Data from small animal models suggest that glomerular capillary haemorrhage and other microvascular rupture could occur when microbubbles are insonated at high mechanical index. This could be injurious in specific situations in which such vascular damage would be clinically important such as in the eye and brain. This potential for non-thermal bioeffects exists with all modes, including conventional 2D imaging and 3D methods. The mechanical index provides a useful, albeit very rough, on-screen indicator of the potential for non-thermal effects. Changes have been observed in vivo in mammalian tissue models for diagnostic ultrasound exposures with a mechanical index (MI) greater than ~0.4 in the presence of ultrasound contrast agents. In addition, premature ventricular contractions have been described when high MI end systolic triggering is used in echocardiography. Users should balance the potential clinical benefit of the use of ultrasound contrast agents against the theoretical possibility of associated adverse bioeffects in humans.25,26 Microbubble agents have the potential for important further developments. The outer membrane of the microbubbles may be loaded with bioactive molecules (e.g. antibody fragments or peptides), resulting in an interaction with the tissue surface. Such labelled microbubbles can bind to specific molecules within the body, anchoring the microbubble in the target tissue.27 After washout of free-floating microbubbles, imaging of the bound agent can demonstrate the presence of binding sites, allowing molecular imaging. The high sensitivity of contrast-enhanced ultrasound (ability to detect even single microbubbles in vivo) and the possibility of microbubble-specific signal detection make ultrasound a highly competitive technique in the rapidly developing field of molecular imaging. Furthermore, the cavity of the microbubble allows the incorporation of active substances, making microbubbles a vehicle for drug delivery. Microbubble-encapsulated drugs can be injected intravenously and released locally, either by short ultrasound-mediated microbubble destruction or by retarded release after local binding of target-specific microbubbles. Using such drug delivery systems, a high local drug concentration and effect can be achieved with a markedly reduced systemic effect. Microbubbles can also be used to deliver gene fragments to living cells in endothelial and parenchymal tissue. In that case, lipid-coated microbubbles serve as a vector for gene fragments, avoiding the use of adeno- or retroviruses. A series of successful animal experiments have already demonstrated the feasibility of these concepts, although many technical and pharmaceutical issues must be resolved before using microbubble-mediated treatments in humans.
Intravascular Contrast Media for Radiography, CT, MRI and Ultrasound
Introduction
Contrast Media for Radiography and CT
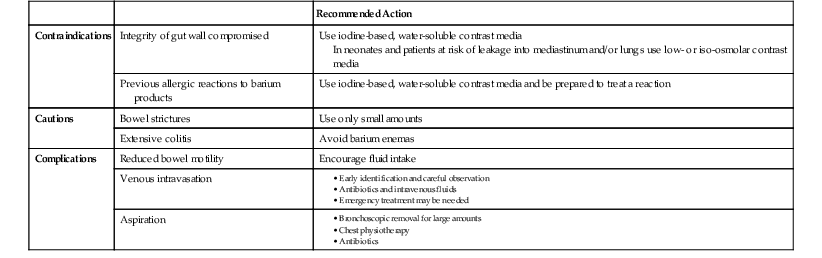
Barium-Based Contrast Agents
Iodine-Based Contrast Media
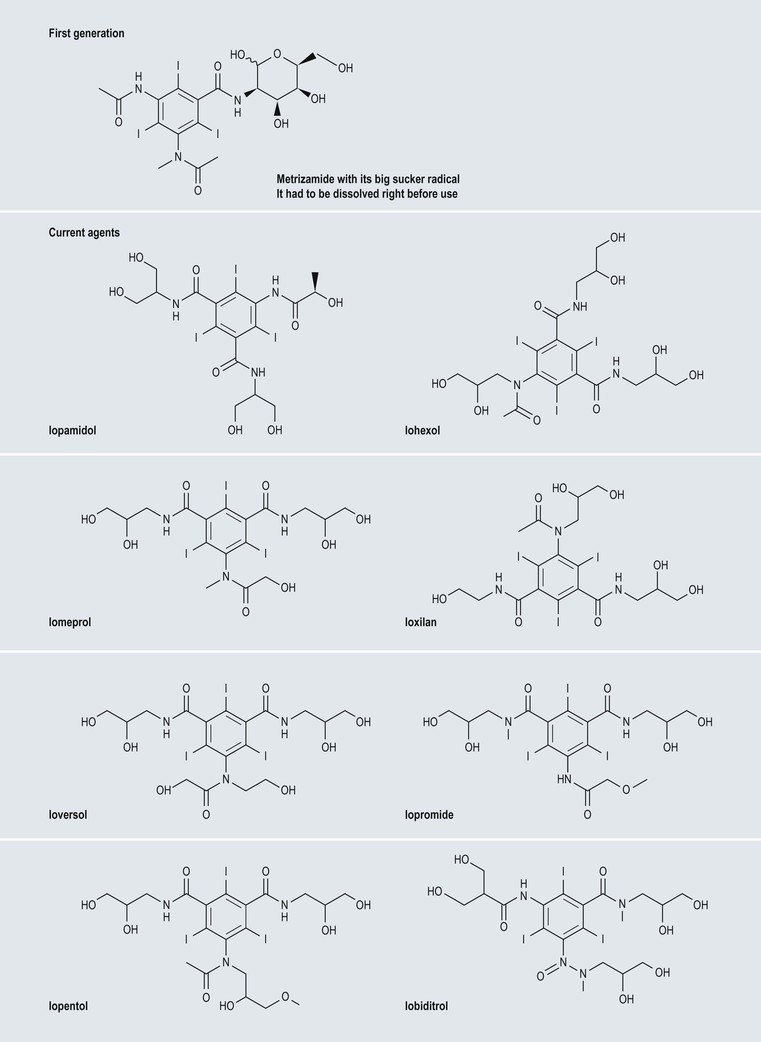
High-Osmolar Ionic Contrast Media (Fig. 2-3)
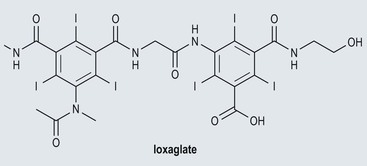
Low-Osmolar Ionic Contrast Media (Fig. 2-4)
Low-Osmolar Non-ionic Contrast Media (Fig. 2-5)
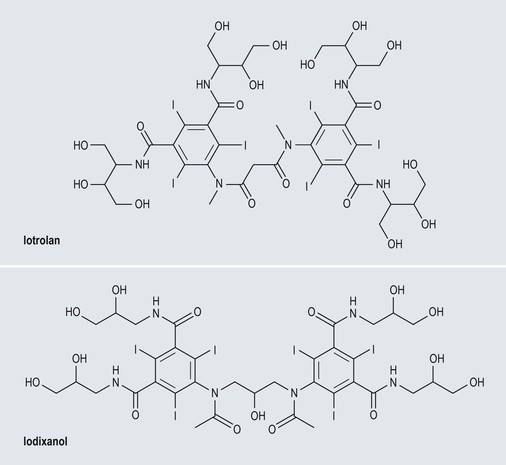
Iso-Osmolar Non-ionic Contrast Media (Fig. 2-6)
Pharmacokinetics
Quantity of Contrast Medium Required
Contrast Media for MR Imaging
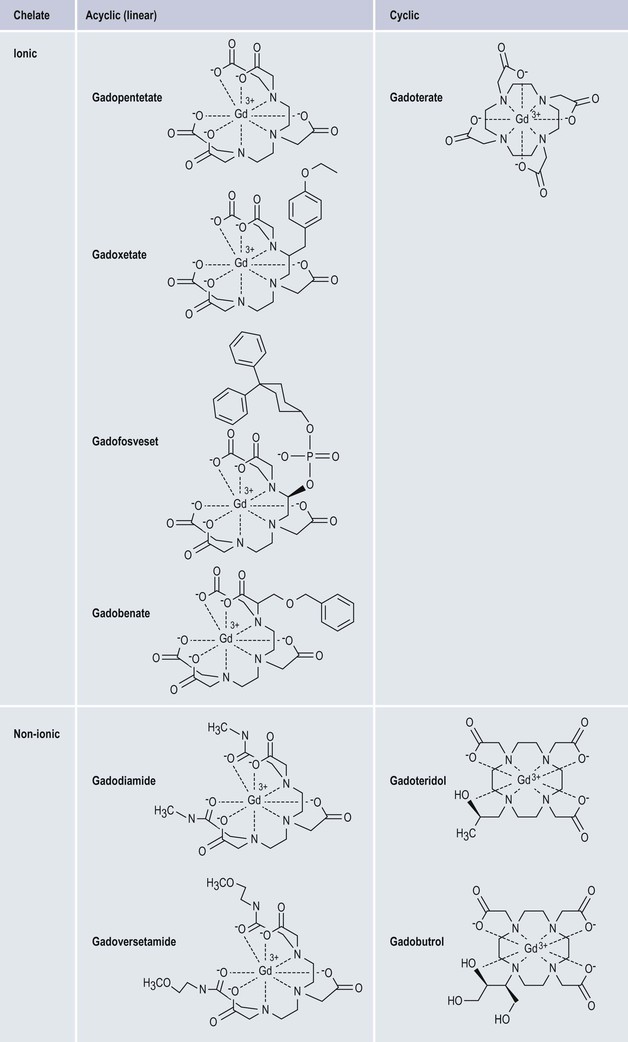
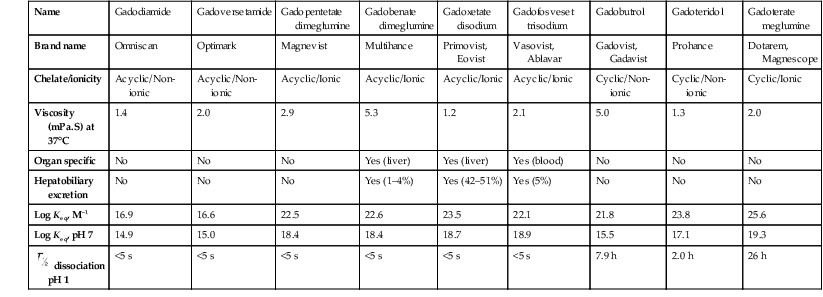
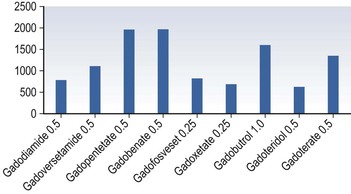
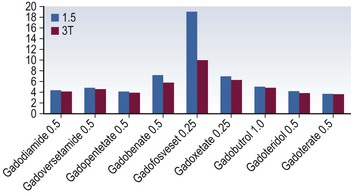
Gadolinium-Based Contrast Media
Pharmacokinetics
Quantity of Contrast Medium Required
Iron-Based Contrast Media
Manganese-Based Contrast Media
Tissue-Specific MR Contrast Agents
Use of Extracellular MR Contrast Agents for Radiographic Examinations
Contrast Media for Ultrasound
Microbubbles
Pharmacokinetics
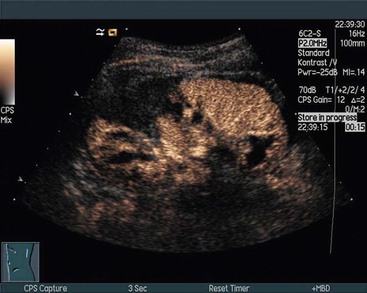
Effect on Echogenicity
Safety
Potential Developments
Intravascular Contrast Media for Radiography, CT, MRI and Ultrasound
Chapter 2