Although mammography is an effective screening test, it is a suboptimal test. A mammogram is a two-dimensional (2D) image (or shadow) of the breast and its sensitivity is limited by breast density. This is a major limitation of screening mammography given that approximately 50% of women in the United States have heterogeneously or extremely dense breasts. In addition, the average tumor size of a cancer detected on a screening mammogram is 1.4 cm and approximately 20% of screen-detected cancers have axillary nodal metastases. Another limitation of mammography is the interval cancer rate; 20% of all breast cancers are interval cancers, meaning that these cancers are diagnosed within 365 days of having a negative mammogram. Interval cancers also tend to be more aggressive and are associated with a worse prognosis. Additional limitations of mammography include the recall rate and false-positive biopsy rate. Approximately 10% of women are recalled after a screening mammogram for further evaluation of a questionable finding; in the majority of cases, no suspicious abnormality is seen when additional mammographic views and/or ultrasound are performed. Finally, when a biopsy is recommended for a mammographic lesion, approximately 70% of those are found to be benign.
Attempts have been made to improve mammographic technology in order to address some of these limitations. These include the conversion from industrial to high-speed screen film, computer-aided detection (CAD) programs, digital mammography, and now digital breast tomosynthesis (DBT). Industrial film was initially used in screening mammography. With time, analog films evolved first to the use of film with grids, then to the use of screen film combinations, and then to high-speed film. The development of high-speed, high-contrast film improved image quality and image consistency. CAD programs were developed in the 1990s in order to highlight areas on the mammogram that may be abnormal. The primary goal of CAD programs is to increase cancer detection, reducing the number of false negatives. Digital mammography was another advancement that was developed in early 2000s. With digital technique, the degree of image contrast can be adjusted to optimize visualization even in areas of dense breast tissue. The Digital Mammographic Imaging Screening Trial (DMIST) was a prospective multicenter trial that included 49,528 women. The trial was designed as a comparative study, with all women undergoing both digital and film mammography in random order. In DMIST, the accuracy of digital mammography was significantly higher than film mammography in women under the age of 50, women with heterogeneously or extremely dense breasts, and in premenopausal or perimenopausal women.
DBT was Food and Drug Administration (FDA) approved in 2011 and is the latest attempt to improve the sensitivity and specificity of mammography. DBT obtains a series of low-dose mammograms at varies angles in order to reconstruct 3D images of the breast. DBT is slowly replacing conventional 2D full-field digital mammogram (FFDM) at many centers throughout the United States. Multiple large studies have demonstrated that when DBT is used in addition to a standard 2D FFDM, the cancer detection rate is increased by 0.7 to 2.7 cancers per 1,000 women. 5, 6, 7, 8, 9 Another benefit of DBT besides increased sensitivity is that the number of women asked to return for additional imaging is reduced by approximately 15% as the tomosynthesis images help decrease unnecessary callbacks due to summation of overlapping normal tissue. 5, 6, 7, 8, 9
Despite these advances in mammographic technique, the cancer detection rate is only slightly improved. Screening mammography still relies on trying to distinguish cancers from the surrounding normal fibroglandular tissue based on a 2D morphological signal or shadow.
Magnetic resonance imaging (MRI) is a vascular-based test not limited by breast density. It has long been known to be the most sensitive test for breast cancer detection, but its use has been restricted to women at the highest risk for breast cancer primarily due to its high cost, time to perform, and perceived low positive predictive value (PPV). However, the landscape of breast cancer screening has changed due to the passage of breast density legislation (BDL) in many states. Many women with mammographically dense breasts are now screened with a combination of both mammography and whole breast ultrasound. An abbreviated breast MRI (AB-MR) that is more sensitive and specific than mammography and ultrasound with a comparable cost may be a more effective way to screen women with dense breasts for breast cancer. However, in order to understand the potential of AB-MR in this new role, it is important to understand the principles of breast cancer screening, what we have learned from BDL, and factors to consider in successfully implementing an AB-MR screening program.
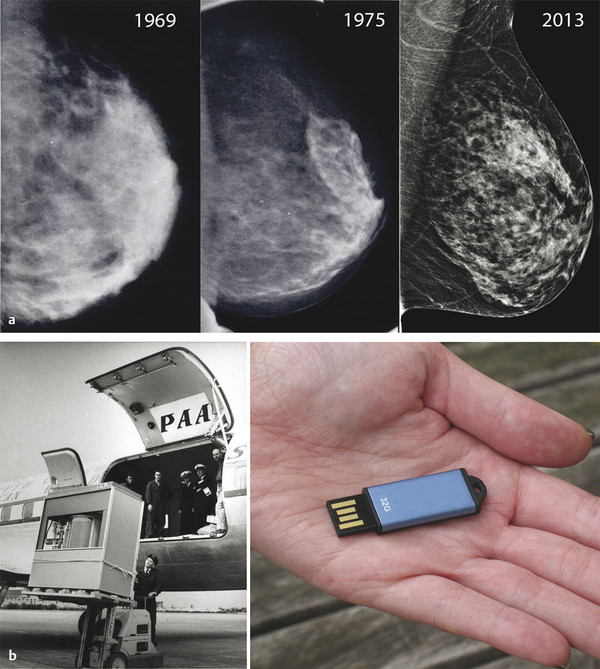
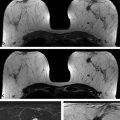
Fig. 1.1 (a) The slow evolution of mammography: left mediolateral oblique views from mammograms performed in 1969, 1975, and 2013. (b) Yet computer memory, for example, has progressed from a 1.5-ton hard drive of 5 megabytes in 1956 (left) to today’s inexpensive USB flash drives with thousands of times the capacity and weighing just a few grams (right).
1.2 Principles of Breast Cancer Screening
Outside of minor fluctuations in breast cancer rates over time due to dietary and environmental factors as well as aging of the population, women develop breast cancer at a relatively constant rate. In the absence of screening tests, these cancers will present as palpable masses once they reach a certain size. The use of a screening test allows some cancers to be detected earlier, at a smaller size before they are palpable. Although the total number of cancers detected remains the same, with screening, the combination of the screen-detected cancers and fewer palpable cancers results in a smaller average tumor size and stage at detection. This translates into a reduction in mortality and the use of less aggressive therapies. The average size of cancers detected and the ratio of screen detected to palpable (interval) cancers is directly related to the performance of the screening technology ( ▶ Fig. 1.2).
Reported sensitivities of a screening test can be very misleading and are dependent on what is being used as the reference standard for the test. For example, although the sensitivity of mammography has been reported as 60 to 80% based on a reference standard of 1 year of follow-up, the true sensitivity based on the number of cancers mammography detects out of the total number of cancers present in the women at the time of the test (reservoir of cancers) is significantly less. A single round of MRI in 1,000 women with a negative mammogram has been shown to find 15 or more additional cancers. Mammography detects approximately 5 to 6 cancers per 1,000 women out of the 20 or so cancers that could be detected with combined screening with mammography and MRI. Therefore, the sensitivity of mammography based on this reference standard is only 30% or less. The reservoir of breast cancers present in women is undoubtedly higher than 20 cancers per 1,000 women. A test that could find cancers at its earliest stage at a size of 1 mm or even less could theoretically reveal a reservoir of cancers on the order of 50 to 100 cancers per 1,000 women ( ▶ Fig. 1.3). This would show the sensitivities of current breast cancer screening tests to be significantly lower than reported.
The first round of screening is referred to as the prevalence screen. On the prevalence screen, more cancers will be found because the screening test will detect cancers in the reservoir which have reached the lower threshold set by the screening test that otherwise would have later presented as palpable masses. On subsequent screening rounds (incidence screens), the cancer detection rate will return to the same rate as it was prior to the institution of screening. The only difference is that once breast cancer screening is instituted, the cancer reservoir will be smaller and subsequent cancers will be detected at a smaller average size and stage, with fewer becoming palpable or clinically evident. Debating the precise benefits of breast cancer screening and rehashing the various randomized control screening trials is beyond the scope of this book. Our assumption and belief is that finding breast cancer at a smaller size and earlier stage will reduce morbidity and mortality from breast cancer, just as screening does for colon, skin, cervical, and other cancers ( ▶ Table 1.1).
Breast cancer screening programs and how they are designed to lower the reservoir of cancers in women are somewhat arbitrary. They depend on the technologies available and what is considered acceptable in terms of cost, time, test frequency, and false positives versus the number and stage of cancers that are detected. In addition, what the scientific community considers appropriate for a breast screening program may not always coincide with what the public wants. This is evident by the proliferation of BDL across many states in which women with dense breasts have demanded the addition of ultrasound to screening mammography in order to detect a few additional cancers despite the relatively poor performance, high cost, and increased false positives of screening ultrasound. Our current approach to reducing the reservoir of breast cancers in women is to combine annual mammography with annual whole breast screening ultrasound (WBUS) which results in an approximate detection rate of 8 cancers per 1,000 average risk women screened. Is there a more efficient way to detect more breast cancers at a smaller size, lower cost, and with fewer false positives? In addition, are there screening technologies that can find cancers that are more biologically significant or harmful?
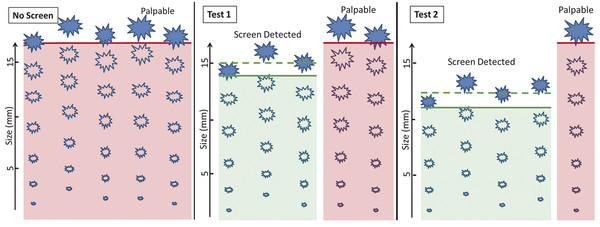
Fig. 1.2 Comparing the performance of two screening tests. In this example, without screening, a total of five cancers develop and are detected only as palpable masses. With screening, some of these same five cancers will be detected by the screening test at a smaller size before they are palpable. With Test 1, three cancers are detected with a mean size of 1.5 cm. However, two cancers are missed on this screening test and will present as palpable interval cancers, resulting in a sensitivity of 60%. Test 2 is a more sensitive test, and detected four of five cancers (sensitivity of 80%). Test 2 also detects cancers at a smaller size than Test 1 with a mean size of 1.2 cm. Only one cancer will present as a palpable interval cancer. Although there are a total of five cancers in both groups, Test 2 detects the cancers at a smaller size with fewer interval (palpable) cancers than test 1. By detecting cancers at a smaller size and earlier stage, Test 2 results in a greater reduction in breast cancer mortality and morbidity.
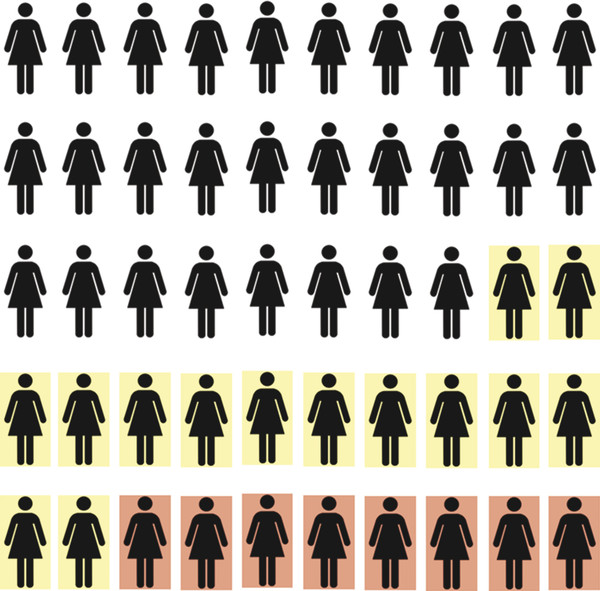
Fig. 1.3 Reservoir of breast cancers. The number of cancers present in 1,000 women being screened at a given time is unknown. Combined screening with mammography and ultrasound will detect approximately 7 to 8 cancers per 1,000 women. An additional 14 cancers will be detected on the first round of screening magnetic resonance imaging.
Tumor size | Relative risk |
Carcinoma in situ | 0.41 |
1–9 mm | 1.0 |
10–14 mm | 1.58 |
15–19 mm | 2.71 |
20–29 mm | 4.88 |
30–49 mm | 8.92 |
≥50 mm | 19.00 |
Adapted from László Tabár, Bedrich Vitak, Hsiu-Hsi Chen, Stephen W. Duffy, Ming-Fang Yen, Ching-Feng Chiang, Ulla Brith Krusemo, Tibor Tot, Robert A. Smith. The Swedish Two-Country Trial Twenty Years Later: Updated Mortality Results and New Insights from Long-Term Follow-up. Radiologic Clinics of North America, Volume 38, Issue 4, 2000, 625–651 | |
1.3 The Current Landscape of Breast Cancer Screening
For over 40 years, mammography has been the primary modality used for breast cancer screening. Screening mammography is the only imaging test shown to decrease mortality from breast cancer. A randomized control trial (RCT) is the most rigorous type of study to determine whether a screening test is effective. In terms of screening mammography, there have been eight major RCTs. In these RCTs, an approximate 30% decrease in breast cancer mortality was seen following the institution of screening mammography. The RCTs have also demonstrated that a mortality reduction is likely to be seen approximately 5 to 7 years after the onset of screening mammography.
The performance and limitations of a screening test are directly related to the physical properties used by the test to detect cancer. One limitation of mammography, since it is based on the penetration and resolution of X-rays to discern structures in the breast based on morphology, is that its sensitivity is affected by breast density. The fifth edition of the Breast Imaging Reporting and Data System (BIRADS) categorizes breast density as almost entirely fatty, scattered areas of fibroglandular density, heterogeneously dense and extremely dense. 10 A woman is considered to have dense breasts if mammographically her density is categorized as either heterogeneously dense or extremely dense. Data from the Breast Cancer Surveillance Consortium (BCSC), which reviewed over 900,000 mammograms, revealed that 46.9% of women have mammographically dense breasts. 11
Breast density is important because of its effect on both the accuracy of screening mammography and its association with increased breast cancer risk. Normal fibroglandular tissue appears dense mammographically, and obscures visualization of cancers, which also are radiodense. The sensitivity of film screen mammography decreased from 85.7% to 88.8% in women with almost entirely fatty breasts to 62.2% to 68.1% in women with extremely dense breasts. 12, 13 Sensitivity of mammography in women with dense breasts has improved with the development of digital mammography. In the DMIST trial, the sensitivity of digital mammography in women with dense breasts was 70%, compared to 55% in film screen mammography. 14
There is also an association between having mammographically dense breasts and increased breast cancer risk. Boyd et al reported that in 1,112 matched case–control pairs, women with extremely dense breasts were 4.7 times more likely to develop breast cancer than patients with <10% fibroglandular density. 15 Another meta-analysis, which included data from over 240,000 mammograms from 42 studies, reported a relative risk of 2.92 for breasts that are 50 to 75% dense and a relative risk of 4.64 for breasts that are at least 75% dense. 16 In many studies, the relative risk of women with extremely dense breasts is compared to women with almost entirely fat density, which are the two extremes of the population. Only approximately 10% of women in the United States have predominantly fatty breasts and another 10% have extremely dense breasts. When compared to women with average breast density, the relative risk is reduced to 1.2 for heterogeneously dense breasts and 2.1 for extremely dense breasts. 17 Finally, several studies have shown that the association of dense breasts and breast cancer is not due to the masking effect alone. 15, 18, 19
Due to the increasing public awareness of the shortcomings of mammography, in 2009 Connecticut became the first state to pass BDL. Many states followed and breast density notification laws are now in effect in 27 states. These laws require that women be notified if they are found to have mammographically dense breasts. In some states, the legislation also specifies that women be told some or all of the following: that mammography may be less sensitive for breast cancer detection in women with dense breasts, that dense breast tissue may increase a woman’s risk for breast cancer, and that supplemental screening should be considered. However, most states do not mandate insurance coverage of supplemental screening.
These laws impact a very large number of women; more than 50% of women younger than 50 years and at least 33% of women older than 50 have dense breasts. 20 In addition, nearly 65% of women in the United States live in states that have enacted BDL. Although the density legislation does not mandate the specific type of supplemental screening test women should consider, WBUS has become the default supplemental screening test. WBUS is a relatively inexpensive test to perform, widely available, and familiar to patients. This has led to a dramatic increase in the utilization of breast ultrasound. The primary advantage of WBUS is that it detects additional cancers that are mammographically occult in 2 to 4 per 1,000 women. Most cancers detected on WBUS are invasive, node negative cancers that are approximately 1 cm in size. However, significant limitations of WBUS include a recall rate of up to 20% and a PPV of approximately 8%. 21, 22, 23 Therefore, 12 benign biopsies are performed before 1 cancer is detected on a screening breast ultrasound. WBUS is also time-consuming for technologist and radiologists and leads to a significant number of short-term follow-up recommendations. WBUS in women with dense breasts has also been shown in modeling studies not to be cost-effective, substantially increasing health care costs while providing small benefits. 23
The landscape of breast cancer screening is no longer based solely on mortality data from RCTs and academic assertion on what is acceptable in terms of time, cost, and possible harms of screening, but also on grassroots movements and public opinion on what efforts should be taken to find a woman’s breast cancer at its earliest stages. Because of this, for many women, the current landscape of breast cancer screening has evolved from annual mammography to a program of annual mammography combined with annual WBUS. Now that these new expectations on our screening program have been set forth, our goal is to improve the cancer detection process by reducing the inefficiencies, cost, and false positives while finding biologically significant cancers at a smaller size and stage and reducing interval cancers.
1.4 Concept of Abbreviated Breast Magnetic Resonance Imaging
Breast MRI is currently the most sensitive test for detecting breast cancer. Screening mammography detects approximately 4 to 6 cancers per 1,000 women screened. With WBUS, an additional 2 to 4 mammographically occult cancers per 1,000 women are detected. However, several studies have demonstrated that breast MRI can detect a substantially larger reservoir of breast cancers that are mammographically and sonographically occult. For example, in the ACRIN 6666 trial, a screening breast MRI was performed in women with dense breasts. A single MRI performed in women after three rounds of negative screening mammograms and ultrasound detected an additional 14.7 cancers per 1,000. 24 Importantly, the PPV3 of MRI was 23% compared to 9 to 11.7% for WBUS. Another study included 427 asymptomatic women at mild to moderately increased risk for breast cancer and dense breasts. In these women with a negative FFDM and WBUS, screening MRI resulted in an additional cancer yield of 18.2 per 1,000. 25 There were seven invasive cancers (64%) and four cases (36%) of ductal carcinoma in situ (DCIS). The invasive cancers detected were small T1, node negative cancers, and both the invasive cancers and DCIS were predominantly high-grade tumors. WBUS in the 11 patients diagnosed with breast cancer was negative.
These studies demonstrate that there is a large reservoir of cancers in the breast that are not detected on either screening mammography or ultrasound, and that there is a technology (MRI) available that is able to detect these cancers at a smaller size and stage compared to either mammography or WBUS without sacrificing specificity. However, due to its high cost, time to perform, perceived low PPV3, and limited availability, screening MRI has been restricted to a small select group of extremely high-risk women. How can we expand the use of MRI to benefit more women?
1.5 It Is Time for a Fundamental Change in How We Utilize MRI for Breast Cancer Screening
The performance of breast MRI has not changed significantly in almost 20 years, with standard protocols including a localizer, T2 weighted with or without fat saturation, a T1 precontrast non–fat-saturated, and a precontrast and multiple dynamic postcontrast T1 weighted sequence. In general, the scan time of a typical breast MRI ranges from 20 to 40 minutes, and patients are scheduled in 45- to 60-minute slots. As currently used, it would not be feasible to expand the role of breast MRI to screen a broader population, such as all women with dense breasts.
What would make MRI screening more feasible? MRI would need to be more efficient with a similar cost and time to perform compared to current practice (e.g., mammography and WBUS in women with dense breasts). Given reimbursement rates in general, a breast MRI would need to be performed within approximately 10 minutes to be comparable in cost to a WBUS. The concept of an AB-MR is to reduce the time to perform the study by including only the most essential sequences while maintaining sensitivity and specificity. Most of the sensitivity and diagnostic information is captured in the first postcontrast series, including lesion morphology and rate of early enhancement. Removing the additional postcontrast dynamic series primarily impacts evaluation of lesion kinetics. Kinetic information is utilized relatively infrequently, such as when deciding to recommend biopsy or follow-up of a lesion on a baseline MR. A T2 weighted sequence is more valuable in breast MR interpretation to improve specificity on a baseline MR. On subsequent screening rounds after a baseline study, kinetic and T2 signal provide little additional information given development of a new or enlarging enhancing lesion is most predictive of malignancy, irrespective of its kinetics and T2 signal. Therefore, an AB-MR protocol that includes a localizer, a T2 weighted sequence, and a T1 weighted sequence before and after contrast administration would allow sites to expand the use of screening MRI to more women without significantly impacting its performance compared to a traditional full MRI.
Given the poor performance of combined mammography and WBUS in terms of false positives and short-term follow-up recommendations, an AB-MR program may be more cost-effective ( ▶ Table 1.2). The difference in the cost of screening with AB-MR versus WBUS will depend on the local reimbursement rate and the payer mix. With an AB-MR costing in the range of $300 to $400, annual screening with AB-MR would likely cost more than WBUS. This cost may be justified due to the increased cancer detection rate of AB-MR, reduced interval cancer rate, and detection of smaller and earlier stage cancers. As MRI detects more than double the number of cancers found on mammography combined with WBUS, AB-MR may only need to be performed biennially to have similar or better performance to annual screening with combined mammography and WBUS. Biennial screening with AB-MR would cost less than annual screening with WBUS.
MRI also has been shown to preferentially detect intermediate and high-grade cancers, which are more likely to be biologically significant 26 ( ▶ Fig. 1.4). Since screening trials using mortality as an endpoint are impractical due to the time and costs involved, trials of newer modalities such as digital mammography, tomosynthesis, and WBUS have used the number of breast cancers detected as a surrogate endpoint to mortality. However, the biological detection profile of cancers may be more significant compared to just the number of cancers detected. Detecting more biologically significant cancers may decrease mortality while reducing overdiagnosis and overtreatment.
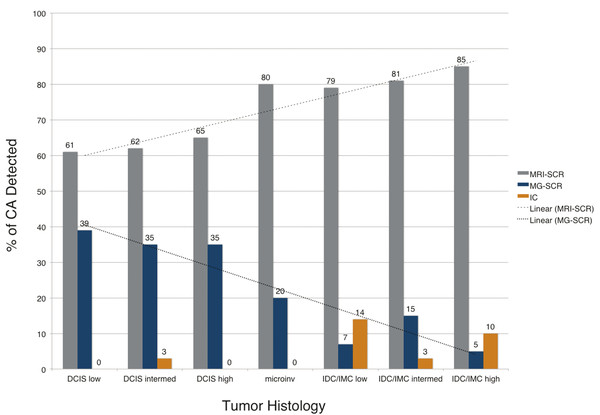
Fig. 1.4 Comparison of tumor histologies by method of detection. Magnetic resonance imaging (MRI) is more sensitive than mammography for all tumor histologies, but especially invasive cancers. More than 80% of intermediate- and high-grade invasive cancers are detected on screening MRI and not mammography in women undergoing combined screening. CA, cancer; DCIS, ductal carcinoma in situ; microinv, microinvasive cancer; IDC, invasive ductal carcinoma; IMC, invasive mammary carcinoma. 26
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree