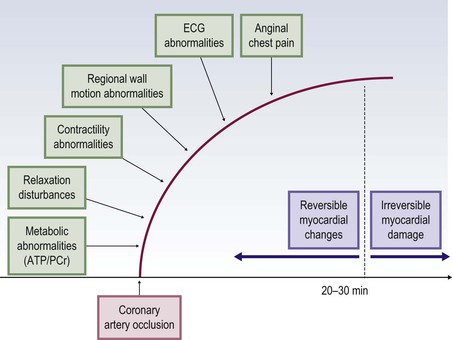
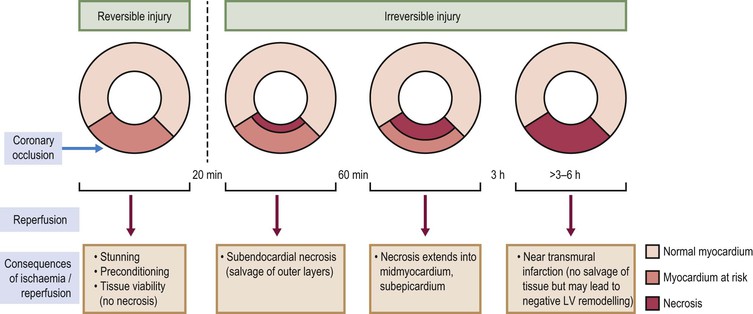
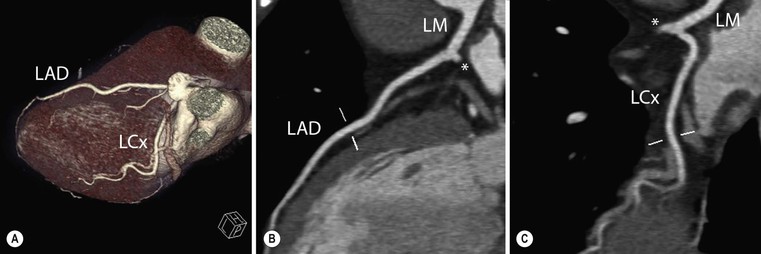
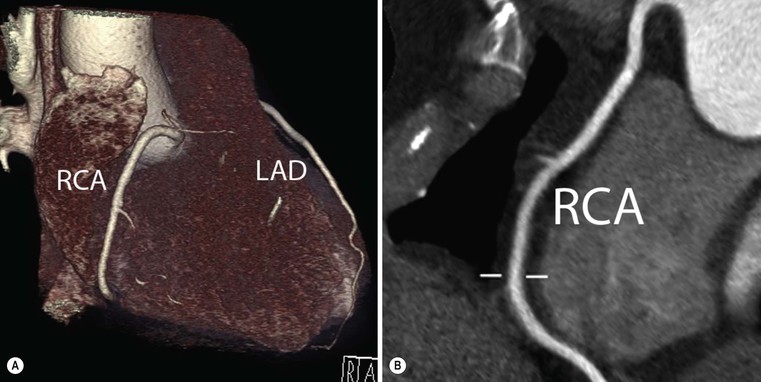
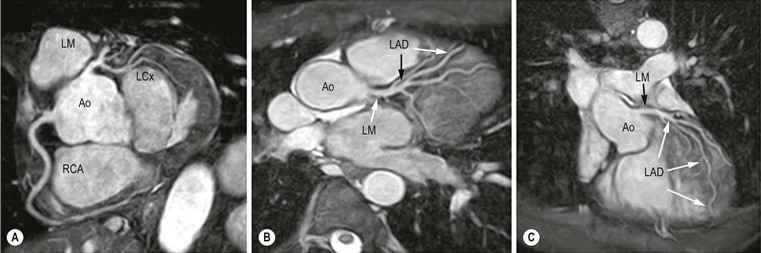
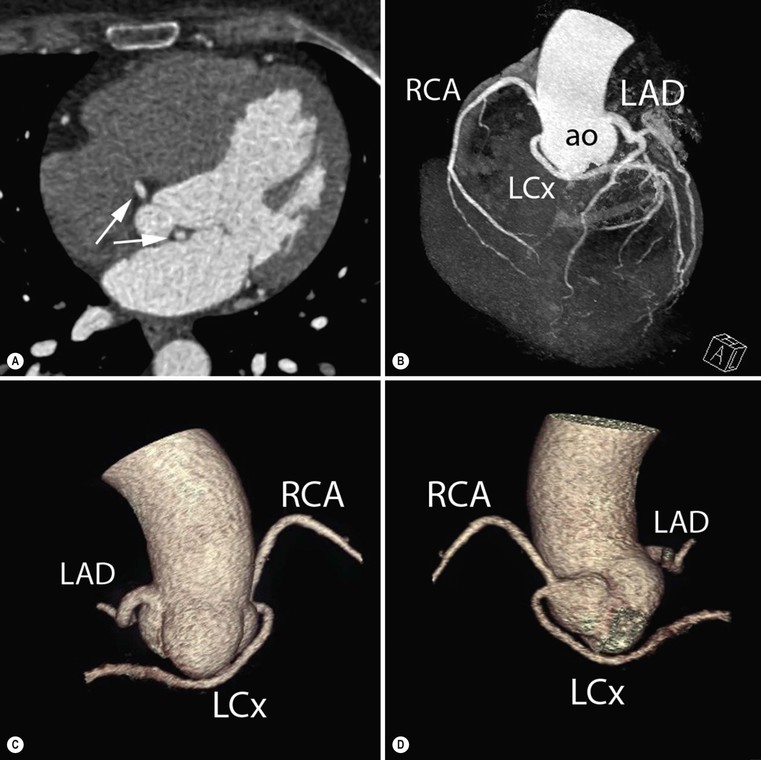
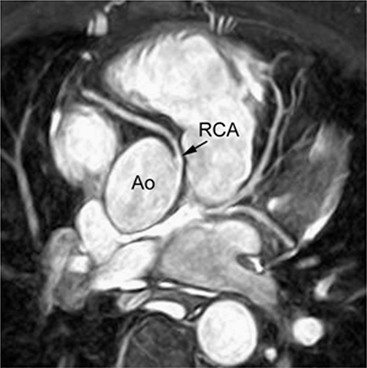
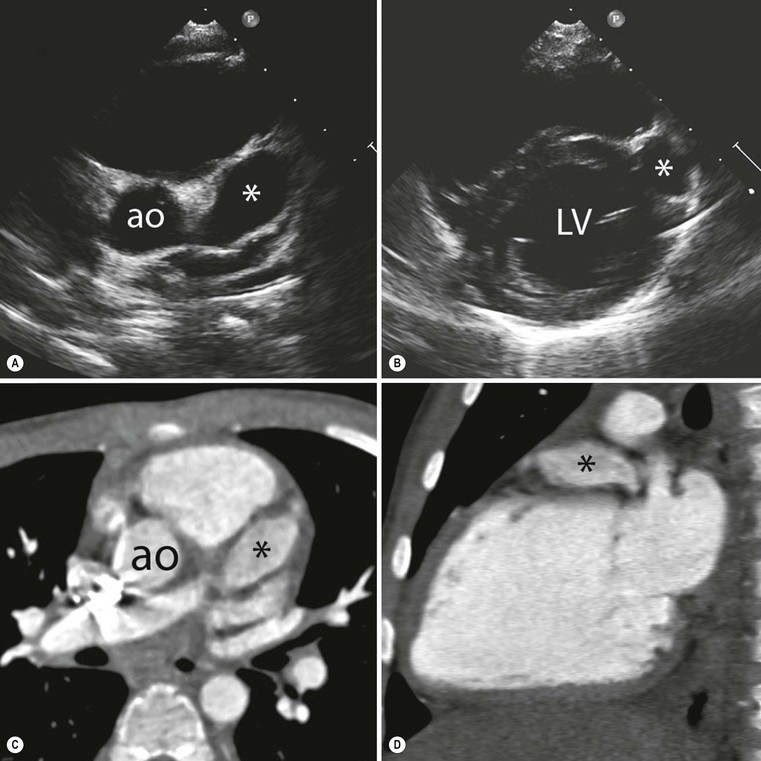
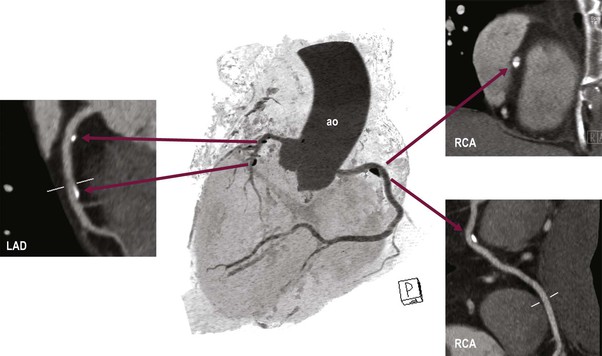
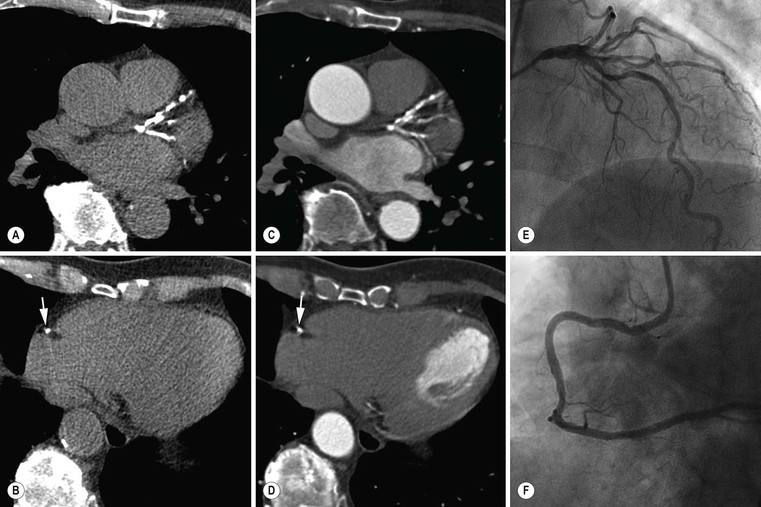
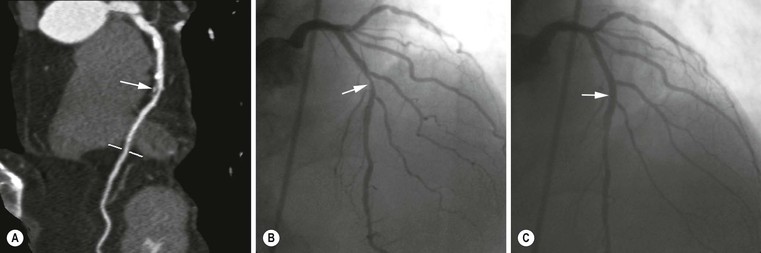
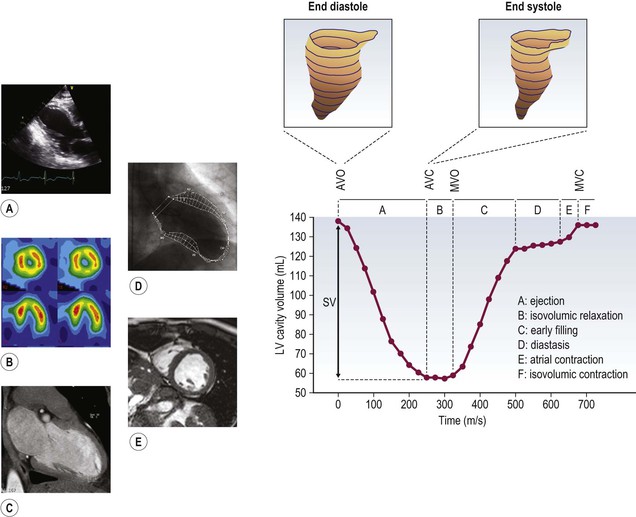
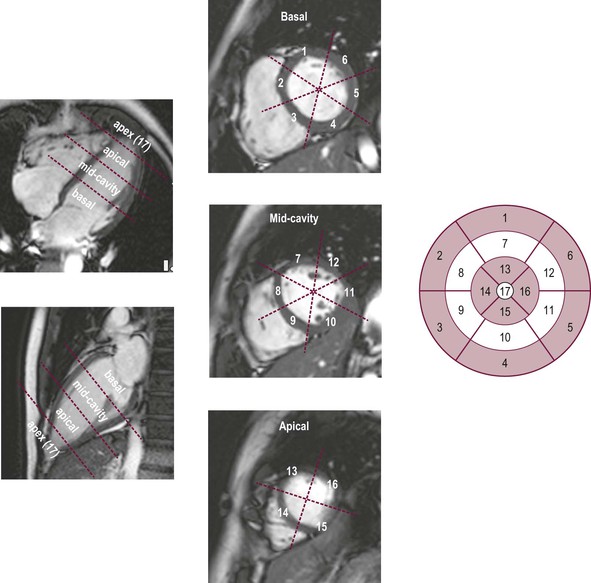
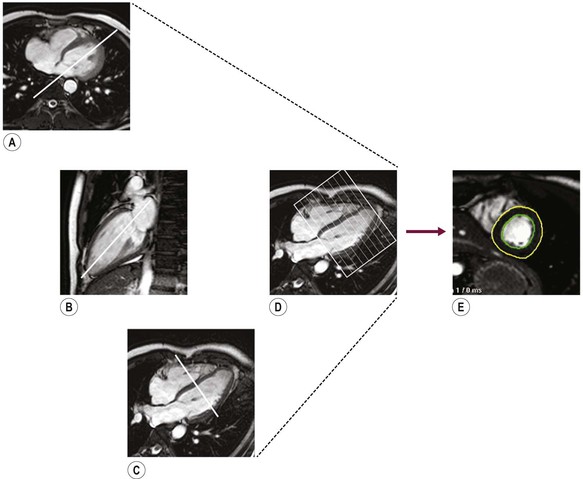
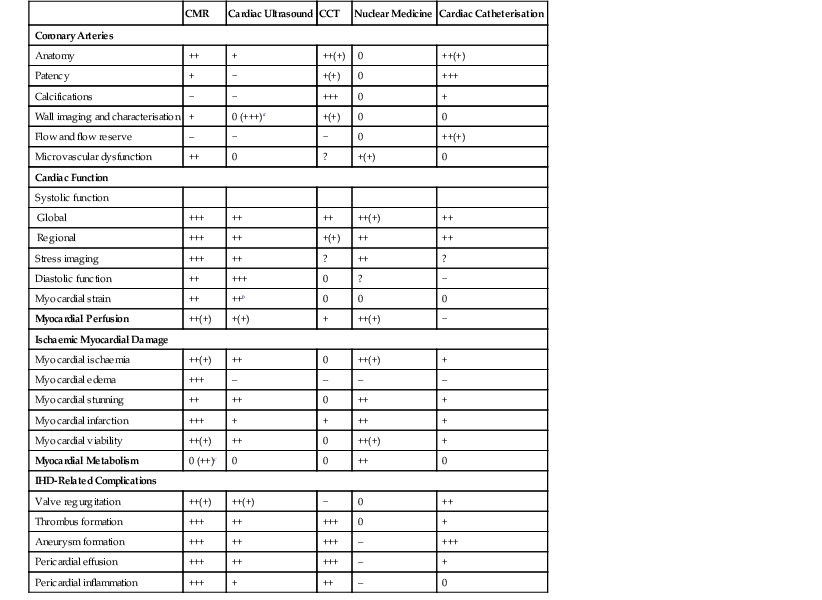
Jan Bogaert Ischaemic heart disease (IHD), the leading cause of morbidity and mortality in developed countries, poses an enormous financial burden on our society. Although the mortality associated with IHD has declined, due to therapeutic improvements and to prevention campaigns reducing the incidence of fatal and non-fatal myocardial infarction, the prevalence of IHD will continue to increase. Worldwide, it is estimated IHD will become the number one cause of death at the end of this decade. Survivors of a first myocardial infarction are thought to be at increased risk of death from IHD at later ages due to heart failure and late cardiac complications. Other contributing factors are an increasing prevalence of type II diabetes, physical inactivity and obesity. In times of constrained financial budgets, the increasing prevalence of IHD will urge for rational use of diagnostic and therapeutic means. Thus rational, evidence-based use of diagnostic and therapeutic means is needed to provide affordable medicine in the coming years. Cardiac computed tomography (CCT) and cardiovascular magnetic resonance (CMR) have emerged in the past decade as important players in imaging of IHD and in preclinical detection of coronary artery disease (CAD), and are appealing to practioners in the assessment of patient prognosis.1–3 The aim of this chapter is to discuss how imaging techniques, with an emphasis on CCT and CMR, can be used to study the highly complex and heterogeneous disease entity of IHD (Table 22-1). TABLE 22-1 Contribution of Imaging Techniques for Studying IHD Patients a Intravascular ultrasound. b Tissue Doppler imaging. c MR spectroscopy. +++, Excellent; ++, good; +, average; −, poor; 0, not possible; ?, unknown. Coronary artery disease (CAD), i.e. the process of atherosclerotic plaque formation, is the usual cause of IHD. Symptoms of myocardial ischaemia occur when the coronary blood flow is significantly impaired. This may happen when the coronary artery lumen is slowly and progressively impinged by an evolving atherosclerotic plaque (chronic stable plaque), or when a coronary artery plaque ruptures (or less frequently plaque erosion) and a thrombus is formed with a sudden occlusion of the lumen causing an acute coronary syndrome (Fig. 22-1). An acute coronary occlusion triggers in the myocardial perfusion territory distal to the occlusion, i.e. the jeopardised myocardium or myocardium at risk, an ischaemic cascade starting with metabolic disturbances followed by regional dysfunction, ECG changes and finally onset of anginal symptoms (Fig. 22-2). Systolic contraction typically ceases within seconds after coronary occlusion. After approximately 20–30 min of sustained ischaemia, irreversible myocardial damage (i.e. myocardial infarction) occurs with myocardial cell swelling, apoptosis, ultimately leading to myocyte necrosis. Cellular necrosis always initiates at the endocardial side of the myocardium with the lateral boundaries of infarction closely corresponding to the myocardium at risk, and follows a transmural wave front progression, taking 3 to 6 hours to reach the subepicardium4 (Fig. 22-3). The amount of necrosis is mainly determined by extent of myocardium at risk and degree of transmural progression. Current therapeutic strategies aim to timely restore coronary flow by percutaneous coronary intervention (PCI) or thrombolysis. This will stop the transmural progression of necrosis, and salvages the ischaemic, but still viable, myocardium. However, despite the beneficial effects of reperfusion, the process of cell death may continue during the first hours of reperfusion, a phenomenon called myocardial reperfusion injury.5 This occurs in the infarct core and is characterised by a lack of reperfusion at myocardial capillary level (i.e. microvascular obstruction or no-reflow phenomenon) despite an effective recanalisation of the infarct-related artery. Myocardial infarctions are usually classified by (a) location (anterior, inferior, lateral), (b) size (focal necrosis, small (<10%), moderate (10–30%) and large (>30% of LV myocardium) and (c) temporally as evolving (<6 h), acute (6 h–7 days), healing (7–28 days) and healed (>28 days).6 Though myocardial infarctions usually affect the left ventricle, extension toward the right ventricle may occur. Isolated right ventricular infarctions, conversely, are seldom. In the days, weeks and months following the acute event, the heart undergoes a remodelling with changes in ventricular size, shape and function with changes not limited to the infarcted myocardium but involving the remote myocardium as well. In an early phase, tissue oedema, haemorrhage and acute inflammation lead to an expansion of the infarct size.4 Eventually this may result in an early ventricular rupture or may rapidly evolve toward an aneurysm. During infarct healing an opposite phenomenon occurs, with a progressive replacement of the necrotic myocardium by a collagen-rich fibrous scar causing a thinning of the affected myocardial wall.4 Depending on the extent of the infarct, part of the ventricle loses contractile force, and as compensatory mechanism usually the ventricle dilates to maintain stroke volume. This, however, is a potentially adverse event, because it may trigger the evolution towards a dilated ischaemic cardiomyopathy and ultimately ischaemic heart failure. If the duration of coronary occlusion is brief (<15 min), no myocardial infarction occurs but recovery of the myocardial dysfunction is typically delayed and is closely related to the length of ischaemia, a condition known as stunned myocardium.7 This is typically encountered in patients with stable or unstable angina, and coronary vasospasm. When a milder degree of ischaemia persists for a longer time, myocytes become chronically dysfunctional by downregulation of energy consumption through a lower level of aerobic and/or anaerobic metabolism. If perfusion is restored to these dysfunctional areas, function may return to normal, although the recovery is typically slow, taking up to more than one year in severe forms.8,9 This condition, known as hibernating myocardium,10 should be differentiated from chronic myocardial dysfunction caused by irreversibly damaged myocardium. Patients typically present with moderate to severely reduced ventricular function, presence of several stenotic lesions on their coronary angiogram and symptoms of heart failure. Because of the cost of revascularisation procedure and the inherent risk related to these interventions, in particular when performed in patients with poor cardiac function, it is crucial to determine preoperatively the benefit of a revascularisation procedure.11 It should be emphasised IHD patients may present with a mixture of different ischaemic substrates (ischaemic, stunned, hibernating, necrotic/scarred myocardium) urging for accurate myocardial tissue characterisation in order to choose the best therapeutic option. The process of atherosclerotic plaque formation usually involves the epicardial part of the coronary artery system. The diagnosis of CAD is made on conventional coronary angiography by impingement and narrowing of the coronary artery. Significant CAD is considered in the presence of a diameter stenosis of ≥ 70% in a major vessel or ≥ 50% in the left main, and usually results in referral for intervention. Though coronary angiography provides valuable information regarding the severity and length of stenosis, CA occlusions, number of vessels affected, stenosis configuration (smooth, ulcerated), presence of thrombus, collateral vessels, CA anatomy and variants, this technique faces several limitations. Mild or non-stenotic CAD is not visualised and no information is provided regarding the plaque composition, or degree of vascular remodelling. Thus, a normal coronary angiogram does not exclude CAD, and a stenotic plaque may be just the tip of the iceberg in some CAD patients. It should be emphasised that in the majority of patients presenting with an acute myocardial infarction the event is caused by rupture of a non- or minimally stenotic plaque. Because of its invasive nature, the need to administrate iodinated contrast agent and radiation issues, use of conventional coronary angiography should be limited to symptomatic patients with high pre-test likelihood of obstructive CAD. Finally, the relationship between myocardial ischaemia and CAD is complex, and many patients fulfilling the criteria of significant CAD turn out not to have a flow-limiting stenosis when measuring the fractional flow reserve (FFR, the ratio of maximum blood flow distal to a stenotic lesion to normal maximal flow in the same vessel). While treatment should be reserved for patients with myocardial ischaemia, the oculostenotic reflex yields the risk of overuse of revascularisation.12 Oculostenotic reflex refers to the tendency to overestimate the functional importance of intermediate coronary artery lesions. Other issues such as collateral vessels and number and length of plaques should be considered in the decision-making. In a minority of patients presenting with typical anginal chest pain and ST-segment depression on exercise testing, no abnormalities are found on conventional coronary angiography. In these patients diffuse subendocardial perfusion defects have been reported on stress perfusion imaging, suggesting that microvascular dysfunction (‘syndrome X’) is the cause of myocardial ischaemia and anginal chest pain. Thanks to technological advances in the field of CCT and CMR, non-invasive coronary angiography has become a reality and these novel techniques get integrated into daily clinical care.13 Current state-of-the-art multidetector CT (at least 64 or more slices) affords coronary artery imaging with sufficient spatial and temporal resolution for clinical use. A typical clinical examination consists of an unenhanced CCT for detection and quantification of coronary calcium, and a contrast-enhanced CCT for coronary artery imaging, detection of coronary artery plaques and, to some extent, characterisation of the non-calcified plaques (Figs. 22-4 and 22-5). Patients are optimally suited if they have a regular heart rate and rhythm, a body mass index below 40 kg/m2 and a normal renal function. The examination is performed following intravenous injection of contrast agent. Image quality can be substantially improved by lowering the patient’s heart rate to < 65 bpm, which is usually achieved by administering β-blockers.13–15 Coronary vasodilatation can be achieved using sublingual nitroglycerin administration. With the newest-generation imaging, not only can the heart be imaged in less than one heartbeat but also the radiation can be substantially reduced (~0.7–3 mSv). Further reduction in radiation dose can be achieved with iterative reconstruction algorithms.16 Coronary MR angiography is achieved using high-resolution imaging targeted to each coronary artery separately, or using a whole-heart approach. Images are acquired during repeated breath-holds or during free breathing using a respiratory trigger algorithm (Fig. 22-6). Sequences are available for luminal and for wall imaging. Despite enormous efforts of CMR experts worldwide, coronary MR angiography is at present still not incorporated in daily clinical care, mainly because of long acquisition times, unreliable image quality and the availability and ease of use of CCT. However, clinically interesting indications for coronary MR angiography are imaging of congenital anomalies of the coronary arteries, coronary artery imaging in (postoperative) patients with congenital heart disease and diagnosis (and follow-up) of patients with coronary aneurysms in Kawasaki’s disease (Figs. 22-7–22-9). Calcification in the coronary arteries occurs, with exception of patients with advanced chronic kidney disease, almost exclusively in patients with coronary atherosclerosis (Fig. 22-10). Since the amount of coronary calcium roughly correlates to the atherosclerotic plaque extent, detection and quantification of coronary calcium is of interest for patient risk stratification.13 Most patients with an acute coronary syndrome (ACS) show coronary calcium, and the amount of calcium in these patients is substantially greater than in age- and gender-matched subjects without CAD. It should be emphasised that coronary calcification is not related to plaque (in)stability and only weakly related to the severity of luminal stenosis (Fig. 22-11). In young symptomatic patients, a negative coronary calcium image does not exclude coronary artery stenoses. The Agatston score, less frequently volume or mass scores, is used to quantify the amount of calcium. Reference data sets stratified by age and gender are available for interpreting coronary calcium shown by CT. In the 2010 Appropriate Use Criteria for CCT, coronary calcium scoring was considered appropriate in asymptomatic low-risk patients without known CAD and with a family history of premature IHD, and for stratifying intermediate-risk patients for future cardiac events.14 Moreover, coronary calcium scoring is of value on the decision to perform the use of contrast CT angiography in symptomatic patients. Above a CCS > 400, a contrast CT angiography is considered uncertain or inappropriate.1 Coronary CT angiography offers high accuracy for detection and especially for ruling out significant CAD (Fig. 22-12). Two recent multicentre trials reported a sensitivity of 95–99%, specificity of 64–83%, negative predictive value of 97–99% and a positive predictive value of 64–86% to identify patients with at least one coronary artery stenosis among individuals at low to intermediate risk for CAD.13 The lower positive predictive value is explained by the tendency to overestimate the degree of stenosis by coronary CT angiography. In particular, calcified plaques tend to be overestimated by the so-called blooming artefact. Coronary CT angiography performs best in symptomatic patients with a low to intermediate likelihood of CAD. In these patient groups, according to the 2010 Appropriate Use Criteria for CCT, coronary CT angiography was deemed appropriate for detecting CAD in symptomatic patients without known heart disease, and also in those presenting with a clinical suspicion of ACS but having normal ECG and cardiac biomarkers, uninterpretable/non-diagnostic ECG or equivocal biomarkers. Coronary CT angiography yields promise to determine and to quantify the coronary plaque burden, and to a certain extent to characterise the plaque composition. Using intravascular ultrasound (IVUS) as reference technique, lipid-rich plaques yielded mean attenuation values between 11 and 99 HU versus 77–121 HU for fibrous plaques.13 Finally, CCT is excellent for depicting anomalous coronary arteries and myocardial bridging.17,18 Assessment of cardiac function is essential in the diagnostic work-up of IHD patients. For instance, in patients admitted with an acute myocardial infarction, the dead myocardium ceases to contribute to the expulsion of blood, shifting the workload to the non-affected (‘remote’) myocardium. Though a compensatory increase in contractility may occur in these areas, the net result is usually a decrease in (global) ventricular functional performance. Several imaging techniques can be applied for functional cardiac imaging, e.g. echocardiography, CMR, nuclear medicine (planar radionuclide ventriculography, gated blood pool single photon emission computed tomography (SPECT) ), catheter angiography and CCT. Requirements are that techniques are accurate, reproducible and preferably non-invasive. Global ventricular function is usually assessed by measuring the volume at end diastole (maximal filling) and at end systole (maximal emptying) (Fig. 22-13). Subtracting both volumes yields the amount of blood expulsed by the ventricle (i.e. stroke volume). Dividing the stroke volume by the end-diastolic volume yields the ejection fraction, while multiplying the stroke volume with the heart rate yields the cardiac output. Moreover, these values can be indexed to body surface area or body weight. Two approaches can be used for this purpose: (a) assumption techniques comparing the ventricle to a geometrical model, and (b) volumetric techniques. Since ventricles have a complex anatomy, their volumes can be quantified using the slice-summation technique; i.e. the ventricle is cut in a set of continuous slices, and the volume (and volume changes during the cardiac cycle) of each slice is (are) quantified. Summing the volumes of these slices yields the ventricular volume. Volumetric quantification is more accurate and reproducible than assumption techniques, at the expense of a more time-consuming analysis. Regional ventricular function is assessed either visually or (semi-)-quantitatively. Ventricular wall motion during systole is usually visually graded as normokinetic, hypokinetic (decreased but still present), akinetic (completely absent), dyskinetic (wall moving outward during contraction) and hyperkinetic (increased). Systolic wall thickening can be visually graded as normal, diminished, absent, wall thinning or increased. Moreover, it is often expressed as percentage systolic wall thickening (normal values are typically in the range of 40%).19 The American Heart Association has recommended the use of a 17-segment model to express regional functional and morphologic parameters.20 The left ventricle is divided longitudinally in a basal level (segments 1–6), a mid-level (segments 7–12), an apical level (segments 13–16) and an apical segment (segment 17) (Fig. 22-14). Segments 1, 7 and 13 represent the anterior LV wall, and a numbering in anticlockwise direction is followed (viewing the LV from apically). Moreover, segments can be attributed to a coronary artery perfusion territory; e.g. segments 1, 2, 7, 8, 13, 14, 17 typically belong to the left anterior descending coronary artery perfusion territory. Segments 3, 4, 9, 10, 15 belong to the right coronary artery, while segments 5, 6, 11, 12, 16 belong to the left circumflex coronary artery. This relation, however, may vary, depending on the coronary anatomy. The strength of this model is that the segmentation is technique-independent. It should be emphasised that functional abnormalities in IHD are not limited to the left ventricle but may affect the right ventricle as well. As discussed in the paragraph on stress imaging, it can be necessary to evaluate the functional cardiac response during stress conditions. Indications are detection of haemodynamic significant coronary artery stenosis, viability assessment in chronic dysfunctional myocardium and depiction of the presence and extent of stunned myocardium in patients with acute myocardial infarction. Cardiac ultrasound is a first-line technique for assessing cardiac function in IHD patients. It can be performed bedside, provides valuable information regarding cardiac structure and function and allows the visualisation of complications such as aneurysm or thrombus formation post-infarction. Novel techniques such as velocity vector imaging or strain imaging are promising tools for studying myocardial motion and deformation with good feasibility in the clinical setting.21 However, in many patients image quality is suboptimal, and geometric assumptions are used in clinical routine to assess ventricular volume and function. Bright-blood CMR, using the balanced steady-state free-precession sequence, has become one of the preferred techniques for assessing cardiac function. Whereas competing techniques need to administer iodinated contrast material (CCT) or radioactive tracer (isotope ventriculography) for volumetric/functional ventricular imaging, these CMR sequences yield a high intrinsic contrast between blood and surrounding myocardium. To obtain dynamic information, images are acquired at multiple time points during the cardiac cycle. Loading these images in a cine mode enables the appreciation of dynamic phenomena such as myocardial wall motion/thickening or motion of valve leaflets (i.e. cine imaging). Though normally acquired within a series of repeated breath-holds, in uncooperative patients or in patients with atrial fibrillation, non-gated real-time cine imaging is a valuable alternative for estimating the degree of ventricular dilatation and dysfunction, for visualising focal aneurysm formation or for image concomitant valve pathology. Cine imaging is performed along the cardiac axes, using a combination of short- and long-axis planes. If needed, images along other planes such as the three-chamber view can also be obtained. This approach allows a full appreciation of regional ventricular function (Fig. 22-15). Ventricular volumes, mass and function are usually quantified using a stack of short-axis slices encompassing the ventricles. To elucidate the complex mechanisms of myocardial deformation in normal and pathologic conditions, tag lines or grids can be non-invasively imprinted on the myocardium.22 Clinical use has been impeded by the elaborative post-processing, but novel techniques such as fast direct colour-coded strain visualisation using strain-encoded (SENC)-CMR are appealing for stress imaging.23 Merging functional imaging with morphological (infarct/oedema imaging) or perfusion imaging is an interesting pathway for assessing the functional performance in the diseased and normal parts of the heart.23 Cardiac CT is an interesting alternative to CMR for left and right ventricle assessment in patients unable to undergo a CMR study.14,24 The administration of contrast agent should be adapted to obtain enhancement of the right ventricular cavity. It is imperative to mention that techniques for reducing radiation dose such as prospective triggering do not allow for functional imaging since data are acquired during a brief period of the cardiac cycle, usually at mid-diastole. In many hospitals, planar radionuclide ventriculography is an established technique for assessing left ventricular volumes and function. Alternatively, gated blood pool SPECT can be used to assess wall motion and regional ejection fraction.25 In patients with chronic stable CAD, treatment goals are threefold: relief of symptoms and ischaemia; prevention of premature cardiovascular death; and prevention of progression of CAD leading to myocardial infarction, left ventricular dysfunction and congestive heart failure. Management of CAD, however, remains highly challenging as several studies have shown that revascularisation fails to improve mortality over medical treatment in randomised trials.26,27 The explanation of this paradox lies most likely in the poor relation between stenosis severity in diffuse CAD and coronary flow physiology.28,29 Whereas anatomical techniques (conventional coronary angiography, CCT) provide limited information regarding the impact of a stenosis on the coronary flow, stress testing can be recommended to assess the extent of myocardial ischaemia before coronary angiography. Though prospective trials are still lacking, there is substantial evidence that a moderate to severe ischaemic burden greater than 5–10%, with or without angina, is an indication for revascularisation, whereas those patients without clear evidence of myocardial ischaemia likely benefit from an optimum medical treatment (e.g. high-dose statins, risk factor modification) to alter the natural history of CAD.12,30 During cardiac catheterisation the functional severity of a stenosis can be determined by the FFR expressing the maximum achievable blood flow to the myocardium supplied by a stenotic artery as a fraction of normal maximum flow. A normal value is 1.0 and a value of 0.75 reliably identifies stenoses associated with inducible ischaemia. The diagnostic accuracy of FFR is >90%.31 Though FFR may be helpful with patients presenting with diffuse CAD at cardiac catheterisation, in which stenoses may benefit from angioplastic interventions, non-invasive testing for reversible (or inducible) ischaemia is warranted to optimally stratify patients with stable CAD. Non-invasive testing for reversible ischaemia is achieved by stressing the heart and evaluating whether during stress, symptoms of angina, ECG signs of myocardial ischaemia, ischaemia-induced myocardial wall motion abnormalities or myocardial perfusion disturbances occur. Exercise ECG test (EET), although widely used in daily practice, has a low sensitivity (0.44 to 0.92, median 0.63) and moderate specificity (0.41 to 0.8, median 0.77),33 and a normal test does not exclude CAD. In particular, EET is a poor diagnostic test in low-risk populations (such as women) owing to its low positive value in a population with a low prevalence of the disease. The limited accuracy of EET in diagnosing CAD is also due in part to its position near the bottom of the ischaemic cascade. Therefore, diagnosis of CAD may be improved by use of non-invasive tests higher up in the ischaemic cascade than EET, assessing abnormalities in myocardial function or in myocardial perfusion during stress conditions. While we are most familiar with myocardial perfusion scintigraphy and stress echocardiography for these purposes, several other single or hybrid techniques have emerged in the field of stress imaging such as stress perfusion CMR, stress function CMR, stress perfusion CT, combined coronary and stress myocardial perfusion imaging by CCT and hybrid cardiac SPECT/CT or cardiac positron emission tomography (PET)/CT.32–36
Ischaemic Heart Disease
Introduction
CMR
Cardiac Ultrasound
CCT
Nuclear Medicine
Cardiac Catheterisation
Coronary Arteries
Anatomy
++
+
++(+)
0
++(+)
Patency
+
−
+(+)
0
+++
Calcifications
−
−
+++
0
+
Wall imaging and characterisation
+
0 (+++)a
+(+)
0
0
Flow and flow reserve
–
−
−
0
++(+)
Microvascular dysfunction
++
0
?
+(+)
0
Cardiac Function
Systolic function
Global
+++
++
++
++(+)
++
Regional
+++
++
+(+)
++
++
Stress imaging
+++
++
?
++
?
Diastolic function
++
+++
0
?
−
Myocardial strain
++
++b
0
0
0
Myocardial Perfusion
++(+)
+(+)
+
++(+)
−
Ischaemic Myocardial Damage
Myocardial ischaemia
++(+)
++
0
++(+)
+
Myocardial edema
+++
–
–
–
–
Myocardial stunning
++
++
0
++
+
Myocardial infarction
+++
+
+
++
+
Myocardial viability
++(+)
++
0
++(+)
+
Myocardial Metabolism
0 (++)c
0
0
++
0
IHD-Related Complications
Valve regurgitation
++(+)
++(+)
−
0
++
Thrombus formation
+++
++
+++
0
+
Aneurysm formation
+++
++
+++
–
+++
Pericardial effusion
+++
++
+++
–
+
Pericardial inflammation
+++
+
++
–
0
Pathophysiology of Ischaemic Heart Disease
Coronary Artery Imaging
Functional Imaging
Stress Imaging
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Ischaemic Heart Disease
Chapter 22