Time (minutes or hours)
Arterial occlusion
NOMI
Venous occlusion
Reperfusion after arterial occlusion/NOMI
0′
Arterial obstruction
No defect, arterial constriction
Venous obstruction
No defect
1′
Mesenteric edema and venous engorgement
Mesenteric edema and venous engorgement
5′
Pale mesentery
Pale mesentery
Reduction in number and caliber of mesenteric vessels
Reduction in number and caliber of mesenteric vessels
30′
Spastic reflex ileus
Spastic reflex ileus
Fluid between intestinal loops
Fluid between intestinal loops
1 h
Fluid between intestinal loops
Fluid between intestinal loops
Intestinal wall thickening, hyperenhancement, or halo or target pattern on contrast enhancement
Intestinal wall thickening, target appearance, or high contrast enhancement of bowel wall
Absent or diminished contrast enhancement usually indicates transmural infarction
3 h
Edema of intestinal loops
Edema of intestinal loops
Free fluid in abdominal cavity
Hypotonic reflex ileus (gaseous stasis)
Hypotonic reflex ileus (gaseous stasis)
Intestinal wall thinning
Intestinal wall thinning
Absent or diminished contrast enhancement at CT
Absent or diminished contrast enhancement at CT
5 h
Free fluid in abdominal cavity
Free fluid in abdominal cavity
Paralytic ileus (gas-fluid mixed stasis)
Paralytic ileus (gas-fluid mixed stasis)
Paralytic ileus (gas-fluid mixed stasis)
8 h
Wall pneumatosis
After 8 h
Wall pneumatosis, portomesenteric pneumatosis, pneumoperitoneum, pneumoretroperitoneum
Wall pneumatosis, portomesenteric pneumatosis, pneumoperitoneum, pneumoretroperitoneum
Portomesenteric pneumatosis, pneumoperitoneum, pneumoretroperitoneum
A possible approach to image interpretation in AMI should be to assess:
(a)
Vessels, in terms of luminal patency, caliber, and gas
(b)
Loops, in terms of wall thickness, degree of attenuation, content, and gas
(c)
Mesentery, in terms of homogeneity of adipose tissue, number and caliber of mesenteric vessels and mesenteric fluid, and pneumatosis.
28.2.3.1 Arterial AMI
Imaging findings related to the pathophysiology of intestinal vascular injury from failed perfusion without reperfusion, i.e., arterial occlusion or hypoperfusion, provide a triphasic course.
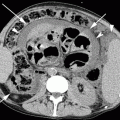
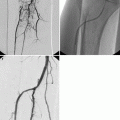
First phase (0–6 h). Mesentery is the first to react, becoming bloodless from severe vasoconstriction (Mazzei et al. 2012a, b). In experimental models, the morphologic changes of the mesentery depend on the appearance of the mesenteric vessels, resulting in reduced caliber and failed perfusion (Berritto et al. 2011). Ultrasound may show an occlusion of the SMA. On contrast-enhanced MDCT images, emboli and thrombi can be seen, if present, as defects in the SMA and its branches (Fig. 28.1a, b).
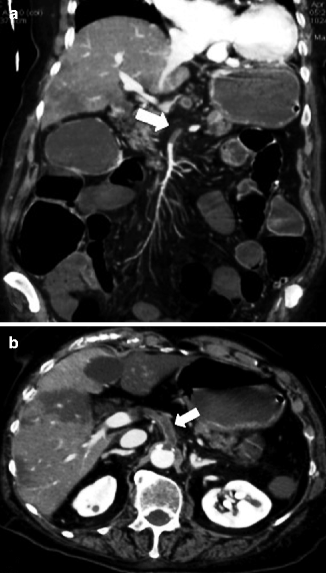

Fig. 28.1
(a, b) MDCT showing thrombus as a defect in the SMA in AMI (arrows)
Response of the intestine to a vascular injury is a spasm of the involved loops (spastic reflex ileus) (Fig. 28.2). Plain abdominal film shows a gasless abdomen. On contrast-enhanced MDCT images, the bowel wall may show normal enhancement, but a thin layer of free fluid between loops may be visible (Romano et al. 2005; Horton and Fishman 2007; Oldenburg et al. 2004).
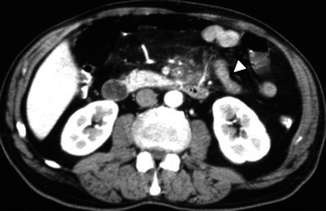

Fig. 28.2
Spastic reflex ileus on MDCT (arrowhead)
Second phase (7–12 h). is characterized by interruption of normal bowel peristalsis and consequent dilation of bowel lumen (hypotonic reflex ileus) defined as a gas-filled dilated small bowel with diameter >2.5 cm.
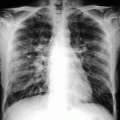
Abdominal plain film may show a mild gaseous dilation of the loops for the total loss of their tone (Fig. 28.3).
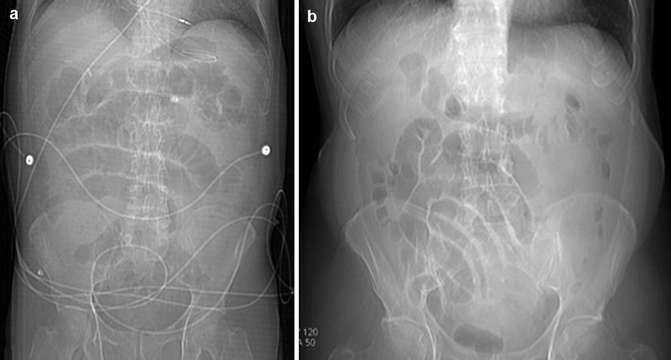

Fig. 28.3
Plain abdominal films: gaseous dilation of the loops for the total loss of their tone in (a) and (b)
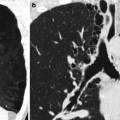
Ultrasound is not informative because of the increased amount of gas in the intestinal loops. MDCT may show all the previous findings, thinning of the involved bowel wall or “paper-thin wall” caused by volume loss of tissue and vessels in the bowel wall and by loss of intestinal muscular tone (Fig. 28.4) (Wiesner et al. 2003; Levy 2007; Horton and Fishman 2001, 2007; Debus et al. 2011; Moschetta et al. 2009).
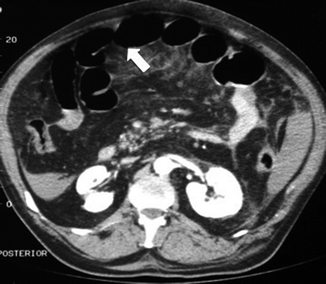

Fig. 28.4
MDCT showing hypotonic reflex ileus with thinning of the bowel wall or “paper-thin wall” (arrow)
On contrast-enhanced MDCT, a highly specific but not sensitive finding for AMI is absent or diminished contrast enhancement of the bowel wall (Klein et al. 1995; Taourel et al. 1996).
In a rat model, non-contrast-enhanced 7T-MR imaging demonstrated a hyperintense signal of bowel wall on T2-weighted sequences, also with a great sensitivity for free intraperitoneal fluid detection. 7T-MR imaging allows hypotonic reflex ileus and lumen dilation to be documented (Fig. 28.5) (Berritto et al. 2011).
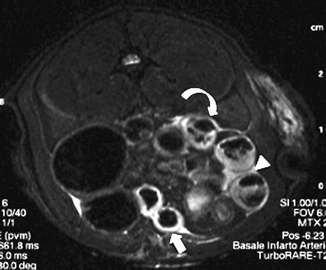

Fig. 28.5
On T2-weighted sequences with 7T-MR imaging, hyperintense signal of bowel wall (arrow), free intraperitoneal fluid (arrowhead), and hypotonic and reflex ileus with gas-fluid mixed stasis can be seen (curved arrow)
In a more advanced phase, plain abdominal film shows a mild dilation of the loops and evidence of fluid- or gas-fluid-only mixed stasis. Ultrasound may show a fluid-filled lumen (Fig. 28.6), evidence of some extraluminal fluid and decreased peristalsis. MDCT may add information regarding occlusion of the SMA and, if present, free intraperitoneal fluid and/or hemoperitoneum (Fig. 28.8), luminal fluid, and the degree of parietal enhancement of the small bowel.
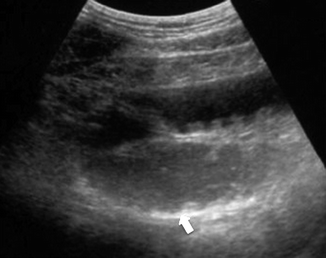

Fig. 28.6
Ultrasound showing a fluid-filled lumen (arrow)
third phase and final phase (12–24 h). Plain abdominal film shows markedly dilated loops and diffuse evidence of air-fluid mixed stasis. Ultrasound shows the presence of extraluminal fluid and the absence of peristalsis. Pneumatosis intestinalis can be indicated when MDCT depicts air in the bowel wall. In the setting of AMI, pneumatosis is a specific indicator but it indicates transmural infarction with advanced bowel necrosis, particularly if it is associated with portomesenteric venous gas (Figs. 28.7, 28.9, and 28.10).
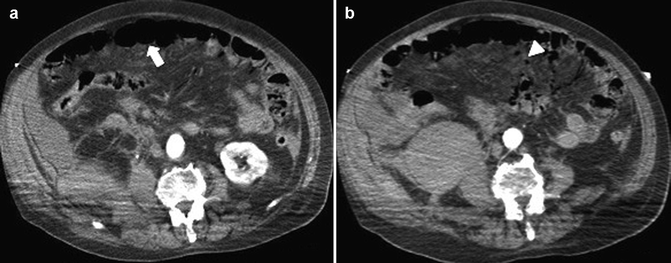
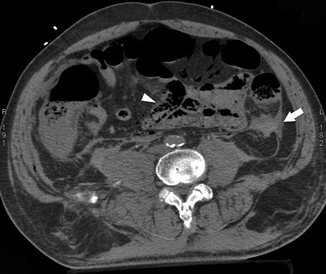
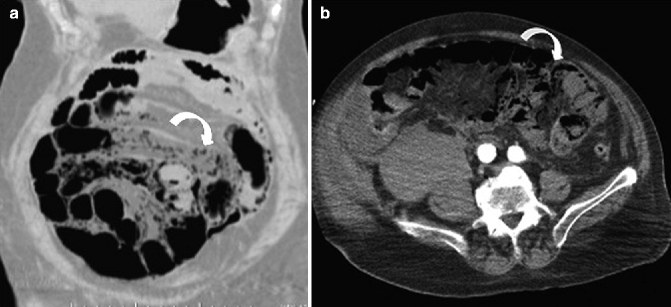
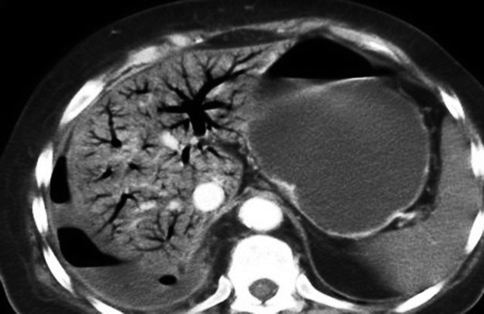

Fig. 28.7
On MDCT hypotonic reflex ileus (arrow) in (a) and mesenteric pneumatosis in (b) (arrowhead)

Fig. 28.8
Hemoperitoneum (white arrow) and bowel wall pneumatosis (arrowhead) visible on MDCT

Fig. 28.9
MDCT showing bowel-wall pneumatosis (curved arrow) in coronal (a) and axial (b) planes

Fig. 28.10
Portal venous gas on MDCT
28.2.3.2 Venous AMI
Impairment of venous drainage causes elevation of the hydrostatic pressure, which leads to extravascular leakage of plasma, RBCs, or both into the bowel wall, mesentery and peritoneal cavity. Tension in the submucosal extravascular compartment or prolonged stasis-induced thrombosis of the microvessels may also compromise the arterial blood flow and cause bowel ischemia and infarction (Furukawa et al. 2009).
The venous vascular injury may be divided into three phases:
First phase. Again, the mesentery is the first to react, immediately appearing congested (Fig. 28.11) because of the elevation of mesenteric venous pressure, with mesenteric vessels increased in caliber.
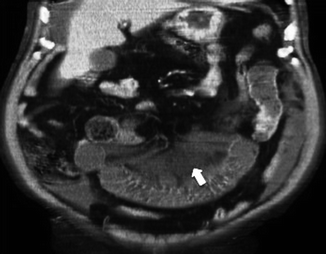

Fig. 28.11
Mesentery engorgement (arrow)
On plain abdominal film, for impaired venous drainage, the visualization of the spastic reflex ileus may not be useful since the congested semicircular folds are unable to contract them.
Ultrasound may show homogeneous hypoechoic intestinal wall due to edema from prior change of the submucosa in intestinal infarction (Kim et al. 1993).
On contrast-enhanced MDCT, thrombus in the mesenteric and portal veins is usually visible, and mesenteric venous obstruction can be confirmed by MDCT in more than 90 % of cases.
MDCT shows homogeneous thickened intestinal tract and the presence of a thin layer of peritoneal free fluid, which increases continuously over time (Rhee and Gloviczki 1997; Romano et al. 2005; Bradbury et al. 2002; Harward et al. 1989) (Fig. 28.12).
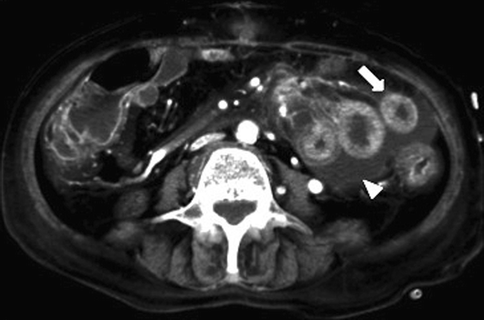

Fig. 28.12
MDCT shows homogeneous thickened intestinal tract (arrow) and the presence of a thin layer of peritoneal free fluid (arrowhead)
Experimental studies on non-enhanced 7T-MR demonstrated a hyperintense signal of intestinal wall associated with a thin layer of peritoneal fluid in the early phase (Somma et al. 2012).
Second phase. On plain abdominal film, a subsequent hypotonic reflex ileus may be masked by progressive intestinal intramural and mesenteric edema; bowel-wall dilation is less related to venous obstruction than in the arterial forms (Romano et al. 2005; Moschetta et al. 2009).
Ultrasound may show a thickened intestinal loop with hyperechoic mucosal layers and hypoechogenicity of the layers due to edema, and occlusion of the SMV at its origin.
The most important MDCT finding is the typical alternating density layers (“target” sign) of the involved intestinal segment: a more evident hyperdense mucosa due to surface hemorrhage, ulceration or superinfection, and hypodense edematous submucosa (Fig. 28.13). It is a specific but the most frequently observed MDCT finding in mesenteric venous infarction (Wiesner et al. 2003; Romano et al. 2005; Horton and Fishman 2007; Lee et al. 2003).
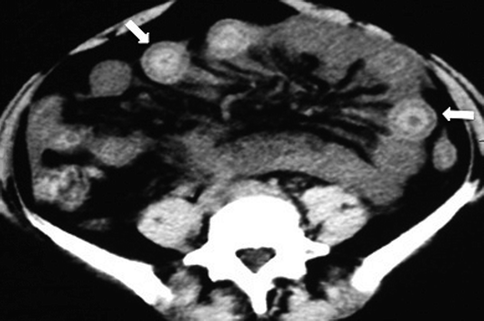

Fig. 28.13
On MDCT typical alternating of density layers (“target” sign) (arrows)
Bowel-wall thickening is also visible on 7T-MR sequences after 2 h from experimental SMV ligation, while after 4 h the main MR imaging findings are dilated loops filled only with gas followed by dilated loops with fluid-gas mixed stasis (Berritto et al. 2011).
Third phase. Progression to intestinal infarction may lead to bowel-wall loss of integrity, and intestinal bacteria may invade the intestinal wall, causing necrosis and peritonitis.
Plain abdominal film may show air-fluid mixed stasis, the thickened bowel wall, and evidence of parietal pneumatosis or pneumoperitoneum. Ultrasound may show a markedly thickened intestinal segment with peristalsis absent and peritoneal inhomogeneous fluid present. MDCT findings are related to evidence of vascular occlusion, peritoneal fluid, and absence of wall enhancement of the intestinal segment involved. Absent or diminished contrast enhancement usually indicates transmural infarction, particularly when it is associated with pneumatosis and portomesenteric venous gas (Rhee and Gloviczki 1997; Romano et al. 2005; Bradbury et al. 2002; Harward et al. 1989).
In experimental models, wall pneumatosis was demonstrated on 7T-MR 8 h after SMV occlusion (Berritto et al. 2011; Somma et al. 2012) (Fig. 28.14); some authors reported that portomesenteric pneumatosis correlates with unfavorable outcomes, while the degree of bowel-wall thickening, mesenteric fat stranding, or peritoneal fluid does not correlate with severity of infarcted bowel damage (Moschetta et al. 2009; Bradbury et al. 2002).
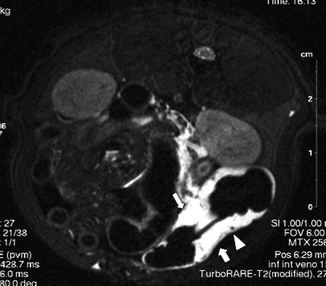

Fig. 28.14
7T-MR imaging demonstrates a hyperintense signal of involved intestinal wall (large arrow) associated with a thin layer of peritoneal fluid (small arrow). A nucleus of wall pneumatosis is also visible (arrowhead)
28.2.3.3 Failed Perfusion Without Reperfusion (NOMI) and Failed Perfusion with Reperfusion
The hypoperfusion deprives both the small and the large bowel of blood; for this reason findings related to the small intestine are the same as the occlusive arterial forms, but these are added to the findings of IC (Mazzei et al. 2013a, b). Furthermore, as in this case critically ill patients are affected, their admission is always early, and it is common to detect the spastic reflex ileus and/or the hypotonic reflex ileus radiologically.
Brief periods of mesenteric ischemia lead to an increase in microvascular permeability, whereas prolonged ischemia leads to disruption of the intestinal mucosal barrier, primarily through the action of reactive oxygen metabolites and polymorphonuclear neutrophils (Schoenberg and Beger 1993; Zimmerman et al. 1990). The reperfused small and large intestine caused by transient arterial insufficiency, due to arterial occlusive or nonobstructive etiology, may have a different pattern, steering the course of veno-occlusive (obstructed outflow) type.
Depending on the degree of microvascular wall damage, blood plasma, contrast medium, or red blood cells may extravasate through the disrupted vascular wall and mucosa, causing congestion of the mesentery, considerable bowel-wall thickening, and bloody fluid filling of the bowel lumen (Chou et al. 2004). Plain abdominal film shows a thickened wall and sparse and subtle valvulae conniventes, while ultrasound may show bowel-wall thickening. On MDCT, the involved bowel segments may thicken and show the halo or target pattern of contrast enhancement (Fig. 28.15) (Wiesner et al. 2003; Levy 2007; Horton and Fishman 2007; Chou et al. 2004; Lee et al. 2003).
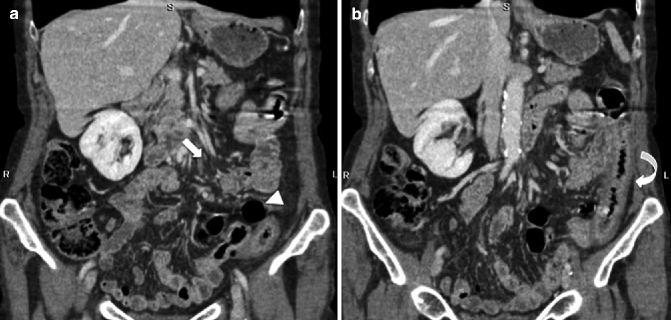

Fig. 28.15
On MDCT, SMA obstruction before reperfusion (arrow) and hypotonic reflex ileus (arrowhead) are present (a); after reperfusion (b) the involved small bowel is thickened and the halo or target pattern of contrast enhancement is visible (curved arrow)
28.3 Chronic Mesenteric Ischemia
Patients diagnosed with CMI are usually over 60 years old; with age and related cardiovascular morbidity, the intestinal arteries become part of the chronic occlusion process (English et al. 2004; Reekers 2009). CMI is characterized by postprandial pain and marked weight loss and is caused by repeated, transient episodes of inadequate intestinal blood flow, usually induced by the increased metabolic demands associated with digestion. It is an uncommon form of AMI accounting for less than 5 % of cases and virtually always seen in association with diffuse atherosclerosis (95 %) (Brandt and Boley 2000; Sreenarashimhaiah 2005).
Although CMI is rare and usually does not require emergency therapy, the risks of incapacitation or acute thrombosis of one of the involved vessels are substantial. The problem with CMI is its progressive nature, which ultimately causes the failure of collateral circulation and fatal mesenteric infarct with high mortality rates. For this reason, evaluation of the intestinal vascular morphology should be part of the repertoire during diagnosis of unclear abdominal situations (Debus et al. 2009).
The large majority of those affected (98 %) suffer from general progressive arteriosclerosis and the corresponding complications (plaque, embolism, arterial thrombosis, dissection). In addition to atherosclerosis, CMI has also been associated with vascular occlusion secondary to fibromuscular dysplasia, Takayasu arteritis, vasculitis, radiation injury, or malignant encasement. Median arcuate ligament syndrome is a form of CMI caused by impingement of the celiac artery by the diaphragm. This syndrome is seen predominantly in women and is more common in younger patients presenting with unexplained abdominal pain.
28.3.1 Presentation
Patients with CMI have a syndrome described as “abdominal angina” by Goodman in 1918 (Goodman 1918) and “intestinal angina” by Mikkelsen in 1957 (Mikkelsen 1957). This syndrome consists of generalized abdominal pain that occurs shortly after meals and persists for 1–3 h. Although it is minimal at first, abdominal pain progressively increases in severity over weeks to months. As with the AMI, the pain relates to an inadequate blood supply, which is incapable of meeting the metabolic demands of the intestine as they increase after eating.
The association of pain with meals leads to fear of eating, with resultant weight loss, which is further accentuated by development of malabsorption seen in up to 50 % of cases. Nausea and vomiting may accompany the pain and in some cases be the dominant symptom (Brandt and Boley 2000; Cangemi and Picco 2009).
28.3.2 Diagnosis
The abdominal plain film, which is usually the first imaging technique employed in suspected cases of CMI, usually does not present any abnormalities. Duplex ultrasonography with B-mode ultrasonography and Doppler waveform analysis is an excellent noninvasive means of accurately detecting mesenteric stenosis in CMI. Patients are easier to examine due to their chronically poor nutritional status. This procedure has an 81 % sensitivity and 96 % specificity. Obscured positioning of the visceral arteries, especially in overweight patients and in those whose stomachs are not empty at the time of the evaluation, can limit the accuracy of the procedure (Armstrong 2007; Atkins et al. 2007).
Non-enhanced MDCT indicate the presence of an existing chronic mesenteric vascular disorder and confirm the existence of aortal and aorta-adjacent calcifications. MDCT angiography may be the procedure of choice for diagnosis of CMI because it is noninvasive, rapid, and offers a more complete examination than conventional angiography. MDCT angiography images distal segments more effectively and can outline the vascular narrowing and the atherosclerotic plaque itself (Ozden and Gurses 2007




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree