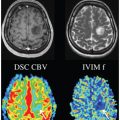
IVIM MRI of the Pancreas
12.1 Introduction
Healthy pancreatic parenchyma is known to be a well perfused. Both inflammatory and neoplastic conditions of the pancreas are characterized by alterations of microstructure, cellularity, and microvascularity.
Since the intravoxel incoherent motion (IVIM) model allows for separate quantification of molecular movement of water (pure diffusion) and microcirculation of blood in capillaries (perfusion) the above-mentioned alterations of tissue architecture are well reflected by the IVIM-derived parameters. IVIM imaging could be a superior method for the characterization and differentiation of different pancreatic diseases compared to the classic monoexponential apparent diffusion coefficient (ADC) model. In the following section, we will address the application of IVIM in different pancreatic lesions and diseases.
12.2 Common Pancreatic Lesions
Pancreatic ductal adenocarcinomas (PDACs), which are the most frequent malignant pancreatic tumors, often appear hypovascular compared to the healthy pancreatic parenchyma in conventional contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI). PDAC is the fourth-most-frequent cause of death from cancer worldwide. The prognosis is poor at the time of diagnosis and is usually made at late stages of the disease due to its unspecific clinical signs and symptoms. In contrast, pancreatic neuroendocrine tumors (PNETs) represent the second-most- common solid pancreatic malignancy and characteristically appear hypervascular compared to normal pancreatic tissue in contrast- enhanced cross-sectional imaging. Chronic pancreatitis and autoimmune pancreatitis are benign pancreatic diseases, which can be difficult to differentiate from malignant adenocarcinomas because of the at-times mass-forming aspect and the often hypovascular appearance compared to the healthy pancreatic parenchyma in conventional CT and MRI.
12.3 Pancreas IVIM Imaging/Acquisition Protocol
Since the pancreas is known to have a well-perfused parenchyma and the first studies using IVIM in pancreas imaging reported high perfusion fractions in healthy pancreas (>25%), the pancreas is a good experimental model for the investigation of the nonmonoexponential signal decay in diffusion-weighted (DW) sequences [1]. Moreover, the dependence on the echo time of f is most important for tissues whose transversal relaxation time is significantly shorter than that of blood. This effect can be expected to be strong for IVIM imaging of organs with short T2 times, like the pancreas (46 ms at 1.5 T [2]). It is classically hypothesized that f is primarily related to the blood flow in the capillary network, which connects the arterial and venous systems [3]. However, animal experiments by Duong and Kim [4] strongly suggest that the fast decaying component at low b values should be attributed mainly to the arterial blood. They reported that the signal contribution from venous blood and capillary blood was minor. Thus, they proposed that f can be described as “apparent arterial fraction.”
Lemke et al. measured the signal in the healthy pancreas as a function of the b values with and without suppression of the vascular component and under varying echo times (TE = 50, 70, and 100 ms) [1]. They found that the perfusion fraction f in the blood- suppressed pancreatic tissue decreased significantly whereas the diffusion coefficient D did not change with the suppression. On the other hand, the perfusion fraction f increased significantly with increasing TE whereas the relaxation time–compensated perfusion fraction f showed no significant dependence on TE. The conclusion was that the steep signal decay at low b values observed in diffusion- weighted imaging (DWI) of the healthy pancreas can be attributed to signal arising from the arterial vasculature. These results make a vascular contribution to the DWI measurement in the pancreas at low b values plausible and support the IVIM theory.
Besides the importance of the applied TE, the number and levels of b values are crucial for IVIM imaging. It is well known that a large number of b values allows for a more stable estimation of IVIM-derived parameters, though at the expense of a prolonged examination time. Examination times of up to 12 min. were reported. Some groups focused on that issue to find the optimal protocol for a reasonable compromise between acquisition time and high reproducibility. The minimum number of required b values is possibly dependent on the fitting method used. Some authors suggested that three-parameter fitting methods require more b values for a reliable derivation of the IVIM parameters than two-parameter fitting methods in which one parameter (mostly D*) is fixed. Koh et al. and Lemke et al. recommended that for three-parameter fitting methods, a minimum of 8 to 10 b values be acquired at 1.5 T for an acceptable variance in IVIM parameter estimates [3, 5].
Recently, Gurney et al. [6] determined the combination of b values for DWI acquisitions at 3.0 T that renders the minimum acquisition time necessary to obtain values of IVIM parameters in the pancreas with acceptable reproducibility. They found that when applying a two-parameter fitting method with fixed D*, 7 b values were sufficient for reproducible IVIM modeling with a low bias. However, their data suggest the use of at least 12 b values when using a three-parameter fitting method (D* not fixed).
To date, there is still no standardized IVIM protocol for the examination of the pancreas across different institutions. Therefore, one should keep in mind that the absolute values of IVIM-derived parameters are difficult to compare between IVIM studies of pancreatic lesions with differing acquisition protocols and different MRI scanners (Table 12.1). The protocols, for instance, differ in terms of the number and levels of b values, TE values, and the use of a motion compensation method (breath-hold vs. free-breathing/ respiratory-triggered sequences). Type and field strength of the MRI scanner also potentially affect IVIM-derived parameters. The number of b values measured in IVIM studies on the pancreas varies from 3 to 11 [5,7–12]. Also, there is no uniform consent on the absolute values of the applied b values. Therefore, absolute values of IVIM-derived parameters from the different studies are comparable to a limited extent only.
Table 12.1 IVIM-derived parameters f and D in healthy/normal pancreatic parenchyma
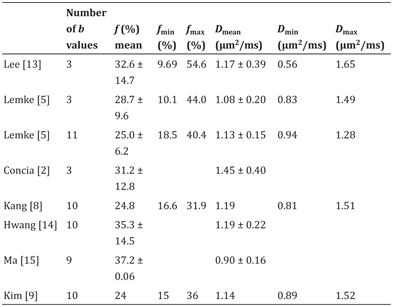
For example, Lemke et al. proposed the use of 11 b values between 0 and 800 s/mm2 to reduce the overall variance in IVIM parameter estimation [5]. b values were separated into blocks (b0, b25), (b0, b50) . . . (b0, b800), and the acquisition was performed using a breath-hold technique. Using this method, they showed that f maps are superior to ADC maps for the visual depiction of pancreatic carcinoma. In contrast to the previous published data of Lee et al. [13] and their own results when estimating the perfusion fraction f using only 3 b values (0, 400, and 800 s/mm2) they found almost no overlap for f between healthy pancreatic parenchyma and PDAC when using 11 b values.
12.4 Pancreatic Adenocarcinoma
Histologically, PDAC is characterized by a complex microarchitecture with abundant desmoplastic stroma, variable microvascularity, and cellularity. The degree of fibrosis, the microvessel density (MVD), and the cell density are known to influence the prognosis of PDAC. Conventional morphological radiological imaging methods are incapable of adequately assessing these histopathological parameters. However, multiple studies have shown that DWI, particularly using IVIM, reflects differences in the microarchitectural properties of PDAC.
An early study focused on the correlation of ADC derived from a monoexponential diffusion model with a histopathology of PDAC and found that the degree of fibrosis rather than tumor cellularity affects ADC in PDAC [16]. However, a recent study by Heid et al. reported a strong negative correlation between tumor cellularity and the ADC [17].
It appears likely that the biexponential IVIM model is superior to a monoexponential diffusion model for assessing the complex microarchitecture of PDAC. One of the first studies investigating IVIM in PDAC was published in 2008 [13]. It showed that D (calculated from b 0, b 500, and b 1000 values) was significantly lower in mass- forming pancreatitis than in pancreatic cancer. The mean perfusion fraction f was useful for differentiating PDAC from mass-forming chronic pancreatitis and normal pancreas, with f values being the lowest in mass-forming chronic pancreatitis and the highest in normal pancreas. These results indicate that the extraction of perfusion effects from DWI measurements by using a biexponential diffusion model may be of additive value for the characterization of solid pancreatic lesions when compared with the use of a monoexponential model of DWI.
Lemke et al. showed f to be the best parameter for differentiation between healthy pancreas and adenocarcinoma, while there was no significant difference of D between the two groups [5]. They reported that the acquisition of 11 b values markedly improved the stability of the IVIM parameter estimation, thus increasing the specificity and sensitivity when compared to the acquisition of only 3 b values [5, 13].
In the following studies with application of IVIM approaches to pancreatic pathology used between 8 and 11 b values for the estimation of IVIM-derived parameters (Table 12.2). In line with the first two studies mentioned above, other studies showed that the perfusion fraction f is reduced in PDAC compared to healthy pancreatic tissue, while D showed no significant difference between healthy and carcinoma tissue [8, 9]. Hence, with the use of IVIM, the perfusion fraction f appears to be the most promising diffusion parameter to distinguish between normal pancreatic parenchyma and pancreatic neoplasms [5,7,13,18], with higher degrees of accuracy than ADC or D.
Table 12.2 Reported IVIM-derived parameters f and D in pancreatic adenocarcinoma
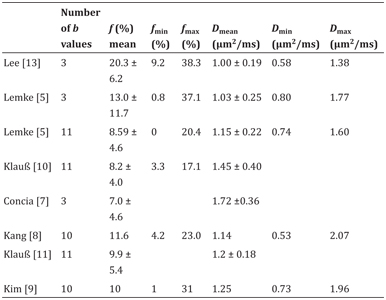
The following studies evaluating IVIM DWI in PDAC indicated in part that this IVIM-derived information about microcirculation offers the potential for better discrimination between PDAC, chronic pancreatitis, and healthy pancreatic tissue as well as between PDAC and PNET [5,10,13,18].
The differences in the perfusion fraction f probably reflect histopathological changes in PDAC. In contrast to normal pancreatic tissue, PDAC is well known for its abundant desmoplastic stroma around tumor cells [19]. In PDAC, activated pancreatic stellate cells produce increasing fibrous stroma in central areas of the tumor, compressing the blood vessels leading to changes in vascularity and perfusion [20]. In a rat animal model, PDAC showed low- density net-like oriented microvessels in contrast to the more highly differentiated architecture of vessels in normal pancreas [21]. This could be explained by a deficient vasculature caused by a dense stromal matrix despite the presence of neoangiogenesis [22–24]. To summarize, PDAC is histologically characterized by a dense desmoplastic reaction around tumor cells, changes in cellularity, and angiogenesis.
Few studies have investigated the relationship between IVIM- derived parameters and histological microvascularity quantitated by MVD in abdominal neoplasms [11, 13] and animal models [25].
One study reported a strong correlation between the IVIM- derived flowing blood volume fraction f and the histologically determined MVD both in PDAC and PNET, which represent the two most common solid pancreatic tumors [11]. There was no correlation between D and D* and MVD in these pancreatic tumors. These findings support the assumption that f reflects the vascularization in pancreatic tumors. The analysis showed that MVD, microvessel area (MVA), and f values in pancreatic adenocarcinoma were lower compared to PNETs, which underlines the match of both histological results as well as previous reports on these parameters in vivo [11]. As mentioned above, stromal reaction with fibrosis is a predominant histopathological feature of both PDAC and chronic pancreatitis [16]. Two studies, both with a comparatively small patient collective, focused on the association of DWI and the degree of fibrosis in PDAC and chronic pancreatitis with somehow contradictory results. Muraoka et al. reported a significantly lower ADC in PDAC with dense fibrosis than in PDAC with loose fibrosis [16]. In contrast, Klauß et al. found that D rises from moderate to severe fibrosis in PDAC and mass-forming chronic pancreatitis [26]. Further studies with larger patient collectives could help to eliminate this ambiguity.
12.5 Differential Diagnosis between Pancreatic Cancer and Chronic Pancreatitis
Various studies investigated the potential of IVIM DW MRI to differentiate PDAC from mass-forming chronic pancreatitis, which is a common problem in clinical daily routine [5,8–10,13,18]. Among the IVIM-derived parameters, f appears to be the best discriminator between these entities, with f being higher in chronic pancreatitis than in PDAC [7,8,10] (Table 12.3).
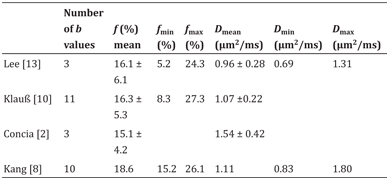
Table 12.3 Reported IVIM-derived parameters f and D in chronic pancreatitis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree