Abstract
The jugular foramen is a complex skull base foramina containing important neurovascular structures that can be directly evaluated only by CT or MR imaging. Thus it is important to have a thorough understanding of the anatomy and anatomic variants of the jugular foramen. This chapter highlights the potential imaging pitfalls and pathologic processes arising from either the intrinsic contents of the jugular foramen or invasion by adjacent structures.
Keywords
Computed tomography, Jugular foramen, Lower cranial nerves, Magnetic resonance imaging
The jugular foramen is a complex bony canal containing neurovascular structures deep in the skull base. It is inaccessible to direct clinical examination and difficult to access surgically because of the surrounding critical structures. Radiology plays a central role in the diagnostic evaluation and management planning of jugular foramen lesions. Consequently, it is important to comprehend the normal anatomy of the jugular foramen, as well as to recognize the anatomical variants and imaging artifacts, which are potential diagnostic pitfalls that may mimic pathology and lead to inappropriate intervention.
Lesions affecting the jugular foramen may arise from its intrinsic contents or from invasion by adjacent contiguous structures. Primary jugular foraminal tumors expand the canal and may extend intracranially, presenting as a posterior fossa or cerebellopontine angle mass, whereas distal lesions that extend extracranially result in a cervical or carotid space mass. Patients often present with otologic symptoms, such as hearing loss and tinnitus, as well as vertigo or ataxia, pain, and lower cranial nerve palsies. Owing to the small caliber of the jugular foramen and close proximity of the nerves within, a space-occupying lesion is likely to impinge on more than one cranial nerve to produce a combination of cranial nerve palsies, which include Vernet syndrome, Tapia syndrome, Schmidt syndrome, Avellis syndrome, Collet-Sicard syndrome, Villaret syndrome, and Jackson syndrome.
Advances in microsurgical techniques have allowed for the resection of jugular foraminal lesions, with improved morbidity and mortality rates. Optimal assessment of jugular foramen diseases preoperatively requires both magnetic resonance imaging (MRI) and computed tomography (CT) with thin-section bone algorithm. MRI shows the exact soft tissue extent of lesions, whereas CT allows precise evaluation of the surrounding bone changes. Angiography may be useful to outline the vascular road map for surgeons or in preoperative embolization for certain hypervascular tumors. This chapter presents an overview of the anatomy of the jugular foramen, including its variants and anomalies, and illustrates the pathologic processes that may involve this foramen.
Applied Anatomy of the Jugular Foramen
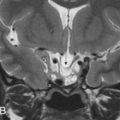
The jugular foramen is an irregular-shaped recess with endocranial and exocranial openings ( Fig. 13.1 ). Embryologically, the jugular foramen is established from the union of the otic capsule and the basioccipital plate, forming a canal-like aperture between reciprocal depressions of the petrous temporal bone and occipital bone. It has a complex oblique course running anteriorly, then laterally, and eventually inferiorly through the skull base into the carotid space. The petrous segment of the temporal bone forms the anterior wall and dome, whereas the jugular process and condylar portions of the occipital bone form the posteromedial margins of the jugular foramen.

There are several important surface landmarks and structures surrounding the jugular foramen. The entry point of the carotid canal is anteromedial to the jugular foramen, separated by the carotid ridge ( Fig. 13.1A ). Lateral to the carotid ridge is a tiny opening, the inferior tympanic canaliculus, where the tympanic nerve courses into the middle ear. Medially, the carotid ridge is contiguous with the jugular spine of the temporal bone. The hypoglossal canal is just inferomedial to the jugular foramen, located above the occipital condyle ( Fig. 13.1B ). Lateral to the jugular foramen is the styloid process, which separates it from the temporomandibular joint. The stylomastoid foramen, where the facial nerve exits, is located just posterior to the base of the styloid process and a few millimeters posterolateral to the jugular foramen.
The cranial nerves within the jugular foramen are difficult to visualize using the conventional MRI protocols. They are best seen on contrast-enhanced three-dimensional fast imaging employing steady-state acquisition and high-resolution contrast-enhanced sequences such as gradient-echo MR angiography (MRA). The steady-state sequence provides high contrast and spatial resolutions using a refocused gradient-echo sequence, whereas enhancement of the jugular vein and venous plexuses further increases the contrast between the hypointense cranial nerves and their surrounding hyperintense venous compartments.
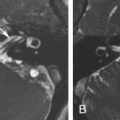
The jugular foramen is divided into three compartments, consisting of two venous (petrous and sigmoid) and a neural (intrajugular) component. The larger posterolateral sigmoid venous compartment drains the sigmoid sinus, whereas the smaller anteromedial petrosal compartment contains the inferior petrosal sinus. The inferior petrosal sinus is the main drainage outlet of the cavernous sinus. It runs along the petroclival fissure into the petrosal compartment of the jugular foramen, where it forms a multichanneled confluence that receives tributaries from the hypoglossal and vertebral venous plexuses. This confluence then empties into the jugular bulb, or less commonly into the internal jugular vein, through openings between the glossopharyngeal (cranial nerve [CN] IX) and vagus (CN X) nerves in the medial wall of the jugular bulb ( Fig. 13.2A ). The posterior meningeal artery also traverses the jugular foramen adjacent to the vagus and accessory (CN XI) nerves to supply the posterior fossa meninges. It is usually a branch of the ascending pharyngeal artery, although it may occasionally arise from the anterior inferior cerebellar artery.

The two venous compartments are separated by a fibrous or bony septum between the jugular spine of the temporal bone and jugular process of the occipital bone ( Fig. 13.2A ). Occasionally, this septum may be completely ossified. The glossopharyngeal, vagus, and accessory nerves exit the cranium through the intrajugular compartment, which is located between the two venous compartments. There are two characteristic perforations in the dural roof of the jugular foramen: the glossopharyngeal meatus where CN IX enters, and inferiorly the vagal meatus through which CN X and XI penetrate ( Fig. 13.2B and C ). The two meatuses are separated by a dural septum.
Upon entering the jugular foramen, CN IX turns forward and downward, forming a genu that is lodged at or near the external opening of the cochlear aqueduct, before coursing inferiorly into the carotid sheath. Extreme care is essential during translabyrinthine surgery to avoid injuring the CN IX genu. The CN IX forms a superior and inferior ganglion within the jugular foramen, with the tympanic branch (Jacobson nerve) arising from the latter. The Jacobson nerve enters the inferior tympanic canaliculus, coursing into the hypotympanum where it forms the tympanic plexus over the cochlear promontory. CN IX is situated anterosuperolateral to CNs X and XI. Upon exiting the jugular foramen, CN IX runs between the carotid artery and the internal jugular vein.
The CNs X and XI complex pierce the dura inferior to the glossopharyngeal nerve. CN X also forms a superior and inferior ganglion within the jugular foramen, with the auricular branch (Arnold nerve) arising from the superior ganglion. This nerve passes through the mastoid canaliculus into the facial nerve canal (mastoid segment) before exiting via the tympanomastoid fissure into the external auditory canal. The CN X and XI nerve fibers intermingle throughout the jugular foramen and are inseparable by microdissection. Within the intrajugular compartment, CN IX is located anterolaterally, whereas the CN X/XI complex is seen posterolaterally ( Fig. 13.2A ).
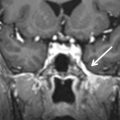
The hypoglossal canal is inferomedial to the jugular foramen, located between the occipital condyle and jugular tubercle ( Fig. 13.1B ). On coronal images, the jugular tubercle has a characteristic “eagle’s head” configuration, which is an important anatomic landmark in locating and separating the hypoglossal canal from the jugular foramen superolaterally ( Fig. 13.3 ). The venous plexus within the hypoglossal canal is referred to as the anterior condylar vein, which joins the inferomedial margin of the inferior petrosal sinus. The hypoglossal canal also contains the neuromeningeal branch of the ascending pharyngeal artery and the hypoglossal nerve (CN CXII). The hypoglossal nerve may consist of one or two trunks, which penetrate the dura to exit the cranium via a single or duplicated hypoglossal canal located above the occipital condyle. CN XII then descends in the carotid sheath together with the glossopharyngeal, vagus, and accessory nerves.

Jugular Bulb Variants and Anomalies
Upon entering the jugular foramen, the sigmoid sinus turns laterally and expands into the jugular bulb. The jugular bulb is usually larger on the right side in up to 75% of individuals. There are a number of important jugular bulb variants and anomalies that should be recognized.
High-Riding and Dehiscent Jugular Bulb
Conventionally, a high-riding jugular bulb is defined as a jugular bulb with its roof or dome extending above the inferior tympanic annulus or floor of the internal acoustic canal. Some authors used the inferior margin of the basal turn of cochlea as the criterion ( Fig. 13.4 ). High-riding jugular bulbs are more common on the right side, with an incidence of 4%–24%. This is an important variant to be mentioned if found incidentally during the evaluation of cerebellopontine angle lesions, as its presence compromises the operative window during a translabyrinthine surgery and precludes adequate exposure of the inferior compartment of the inner ear and the cerebellopontine angle. Modified surgical techniques may be required, such as transotic or retrosigmoid approaches.

A high jugular bulb may be associated with focal dehiscence of the jugular (sigmoid) plate, allowing protrusion of the bulb superolaterally into the posterior hypotympanum ( Fig. 13.5A ). When this occurs, the dehiscent jugular bulb is seen as a bluish posteroinferior retrotympanic mass on otoscopic examination. These patients are asymptomatic, although some may experience pulsatile tinnitus or conductive hearing loss secondary to obliteration of the round window niche or impingement of the ossicular chain ( Fig. 13.5B ). More importantly, it is imperative that surgeons are alerted to this anatomic variant, in view of the high risk of torrential bleeding should a myringotomy or exploratory tympanotomy be performed in these patients.

Jugular Bulb Diverticulum
Jugular bulb diverticula are thought to be more common than previously reported in English literature. The diverticulum is an out-pouching that is often, but not always, from a high-riding jugular bulb. It most commonly extends superomedially into the petrous temporal bone medial to the labyrinth and may be in close relationship with the internal acoustic canal, endolymphatic duct or sac, or the posterior semicircular canal. Some authors believe that it represents a variant or medially located high-riding jugular bulb, which has extended into the petrous temporal bone but is partly hindered by the dense otic capsule. Others considered this to be a true venous anomaly that has enlarged over time.
Jugular bulb diverticulum is usually an incidental finding on imaging for unrelated reasons. However, individuals may present with an air-bone gap or sensorineural hearing loss caused by dehiscence into the posterior semicircular canal or internal acoustic canal, or Meniere syndrome secondary to encroachment of the endolymphatic sac. Jugular bulb diverticula are not visible on otoscopic examination; thus this is essentially a radiologic diagnosis. It is best demonstrated on high-resolution temporal bone CT as a well-corticated polypoid projection extending from the jugular bulb margin ( Fig. 13.6 ).

Jugular Bulb Pseudolesion
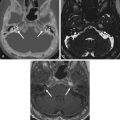
It is common knowledge that both turbulent and slow flow within venous structures result in increased signal intensity on MRI. This is frequently detected in the jugular bulb, especially on the right side in an asymmetrically large and often high jugular bulb. The flow phenomenon results in high or intermediate signal intensity particularly on the T1-weighted images ( Fig. 13.7A and B ), simulating an intraluminal thrombus or tumor. This potential imaging pitfall should be recognized and avoided by radiologists, sparing patients from unnecessary treatment and risk.

By definition, jugular bulb pseudolesions are asymptomatic and found incidentally during MRI. Careful scrutiny of the pseudolesion usually reveals that the flow phenomenon does not hold up on all MR sequences. Further assessment with phase contrast MR venogram or a contrast-enhanced CT or MR is also useful in demonstrating a patent jugular bulb with normal flow enhancement ( Fig. 13.7C and D ), thus excluding an intraluminal lesion or thrombus. On high-resolution CT of the temporal bone, the normal smooth cortical margins of the jugular foramen are preserved with an intact jugular spine.
Vascular Conditions or Lesions
Dural Arterial Venous Fistula
Intracranial dural arteriovenous fistulas (DAVFs) have a direct communication between meningeal arteries and dural veins or venous sinuses. DAVFs in the posterior fossa are more common in the region of the transverse and sigmoid sinuses and rarely around the jugular foramen, with predominantly case reports in the literature. The arterial supply may be provided by branches of the vertebral or external carotid arteries, most commonly the occipital and ascending pharyngeal arteries.
Patients may present with a myriad of clinical signs and symptoms, depending on the location and venous drainage pattern, including the presence of venous reflux. The common symptoms are often nonspecific, such as headache and pulsatile tinnitus. Conventional MRI and CT may show intracranial hemorrhage, venous congestion and edema, parenchymal or leptomeningeal enhancement, and tortuous vascular flow voids. There may be a shaggy appearance of the bony margins on CT bone algorithm because of transcalvarial venous channels ( Fig. 13.8B ). CT or MR angiogram may be used to show enlarged feeding arteries and prominent draining veins with early venous enhancement ( Fig. 13.8A and C ). More recently, time-resolved dynamic MRA techniques have been shown to be more sensitive than time-of-flight MRA in the detection of DAVFs, including in the posterior fossa. However, imaging studies are often unremarkable for small or benign DAVFs. Catheter angiogram remains the gold standard for the definitive diagnosis and assessment of DAVFs.

Treatment options include endovascular, surgical (including stereotactic radiosurgery), or combined management, mainly determined by the location and accessibility of the lesion and skill of the neurosurgeon and interventional radiologist. Management decisions are also influenced by patient characteristics, presence of aggressive features such as venous congestion or hemorrhage, and ultimately the estimated risk of hemorrhage from an untreated DAVF compared with morbidity and mortality from treatment complications.
Jugular Vein Thrombosis
Venous thrombosis of the jugular or cerebral veins is difficult to diagnose clinically and may be missed on initial imaging by the unsuspecting radiologist. The presenting clinical symptoms are usually nonspecific, such as headache or neck pain, seizures, and fever. Risk factors may be present, such as dehydration, coagulopathies, peripartum state, or infection. Thrombophlebitis of the internal jugular vein may occur secondary to local infection (e.g., mastoiditis) or in Lemierre syndrome as a result of hematogenous seeding from bacterial pharyngitis ( Fusobacterium necrophorum ). This in turn may lead to thrombosis of the internal jugular vein and septic embolism.
The appearance on nonenhanced MR or CT depends on the age of the thrombus. In acute thrombosis, a hyperdense clot is seen on nonenhanced CT. Following intravenous contrast administration, there is enhancement surrounding the hypoattenuating thrombus on CT ( Fig. 13.9 ).

On MRI, when normal venous flow void is absent on routine spin-echo sequences, the vein has to be carefully scrutinized for the possibility of thrombosis. This may warrant further evaluation with a contrast-enhanced study or venogram. MR venogram techniques include two-dimensional time-of-flight or phase-contrast study. Slow flow or hypoplasia of the jugular vein can be easily mistaken as thrombosis. In the latter, there is associated hypoplasia of the ipsilateral transverse sinus and usually homogenous enhancement of the veins when contrast is administered. Occasionally, heterogeneous enhancement of the vein on contrast-enhanced T1-weighted images caused by turbulence may resemble central thrombus. As a result of MRI pitfalls, CT venogram is generally regarded as a more reliable modality because it is easy to interpret and is less subject to false diagnosis from the flow-related artifacts on MRI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree