- •
Radionuclide MPI using SPECT or PET techniques is a widely used approach to identify the presence of CAD-related perfusion abnormalities and assess LV function.
- •
There exists an extensive literature supporting the use of these techniques in prognostication, and SPECT and PET have been shown to contribute incremental value over and above clinical historical and stress test information.
- •
MPI’s risk stratification contribution is made through the identification of low-risk patients vis-à-vis a normal MPI study; gradations of increasing risk are identified by the presence of worsening abnormalities of LV perfusion and function.
- •
In addition to the use of LV perfusion and function, the use of PET-assessed quantitative coronary flow and coronary reserve greatly enhance the value of testing and extend its use to the realm of vascular function and atherosclerosis assessment.
- •
Studies examining post-MPI resource utilization yield insights into the appropriateness of post-MPI patient management and how physicians use MPI results.
- •
Although MPI’s clinical application for many years has been in the realm of prognostication and risk stratification, increasing evidence points to a potential role for MPI in identifying which patients may benefit from revascularization after testing.
- •
Although RCTs and their substudies to date have not defined such a role for radionuclide MPI, additional studies with more appropriate designs are needed to better address this question.
- •
Although the area of cost-effectiveness is methodologically controversial and challenging, numerous papers have claimed that the use of radionuclide MPI in appropriate populations is cost-saving compared with other testing strategies.
Introduction
For almost a half century, radionuclide myocardial perfusion imaging (MPI), often using single photon emission computed tomography (SPECT) and, more recently, positron emission tomography (PET), has been a reliable mainstay in the assessment of patients with known or suspected coronary artery disease (CAD). In the current era with its technical advances in coronary computed tomographic angiography (CCTA) and with perfusion, magnetic resonance imaging (MRI), and contrast stress echocardiography entering the clinical realm—as complementary and alternative approaches—the evaluative armamentarium of clinicians has expanded accordingly. These modalities are widely applied to a diverse group of patients with varying presentations, characteristics, and history. Nevertheless, the basis of identifying optimal candidates for testing, as well as the management of patients after testing, is directed by estimating patient risk based on all clinically relevant information and considering the risks and potential benefits of available therapies. In this chapter, the principles of prognostication and risk stratification in the context of radionuclide MPI will be reviewed, and the relevant biomarkers of risk and applicable guidelines and appropriateness statements will be presented. Finally, recent relevant randomized clinical trials (RCTs) impacting cardiovascular imaging and the cost-effectiveness of MPI compared with alternative strategies will be presented.
Risk stratification and incremental prognostic value
Early studies of radionuclide MPI examined the association of imaging results (resting and peak stress perfusion) with posttesting patient outcomes on follow-up. These studies, although relatively small and underpowered, found that the extent and severity of perfusion defects had a significant association with adverse cardiac outcomes. Multiple studies supported the superior prognostic value of both planar and SPECT MPI compared with other information available at the time of testing.
Importantly, these older studies did not address whether the radionuclide MPI prognostic results were still significant after clinical, demographic, and other previously known information about the patient, also known as the incremental value of the patient, was considered. , This approach was first shown by examining the global chi-square of a multivariable model when MPI information was added to a model consisting entirely of pre-MPI data. This approach, first shown in patients undergoing stress-redistribution 201 thallium (Tl), , was later confirmed with SPECT using a dual-isotope approach. Subsequently, an incremental risk stratification was used as well, as demonstrated by successful, successive stratifications initially by pre-MPI data (thus divided into low, intermediate, and high preimaging risk), followed by further risk stratifications by MPI data in each of the subgroups. , Emerging at a time of increasing concerns regarding the costs of testing and issues of cost containment, the concept of incremental value resonated in the noninvasive imaging literature as a paradigm to better demonstrate the value that imaging was providing. Numerous publications that followed demonstrated the added value of MPI compared with demographic, clinical, historical, and stress test information in different patient subsets ( Table 17.1 ).
| Category | Important Markers of Increased Risk |
|---|---|
| Demographic |
|
| Clinical |
|
| Historical |
|
| Stress test |
|
| Data from other imaging modalities | Significant markers from echocardiography, coronary computed tomographic angiography, coronary artery calcium |
Principles of prognostication and risk stratification
Although, in its earlier years, stress radionuclide MPI was clinically used and assessed based on an anatomic end point (i.e., accuracy for identifying obstructive CAD as defined by invasive catheterization), by the 1990s the focus had shifted to prediction of risk for adverse events and risk stratification. This differs from the traditional anatomy-based approach in that the criteria for success is replaced by the value of the test results to accurately define the risk for adverse events. By defining increased risk for untoward preventable events using MPI, the results of testing can be used to guide subsequent management with an eye to reducing patient risk via appropriate therapies and management approaches. A key element in applying this approach to testing is carefully defining thresholds as low, intermediate, and high risk for adverse cardiovascular events. These thresholds were first put forward as part of guidelines defining low risk as an annual cardiac mortality rate less than 1%, intermediate risk as an annual cardiac mortality of 1% to 3%, and high risk as an annual cardiac mortality rate greater than 3%. There are several limitations, however, to defining thresholds of risk in this way. First, with decreasing rates of cardiac mortality over the past 20 years, there is an increasing use of composite cardiac end points for both observational studies and RCTs. Thus these thresholds often cannot be applied to published data. Furthermore, these rates are heavily dependent on the cohort examined. For example, a 1% mortality rate may be intermediate to high risk in a younger population without prior CAD and few risk factors, whereas it may be very low risk in an elderly cohort. Similarly, the applicability of these thresholds to various relevant patient subgroups is unclear (e.g., women, patients with diabetes, patients with prior CAD, and patients with atherosclerotic disease without obstructive lesions).
Based on the extensive available literature examining the prognostic implications of radionuclide MPI, a framework for understanding patient risk assessment emerges. Three general factors drive patient risk after radionuclide MPI (or any test)—the result of the study, the patient’s underlying clinical risk, and the posttest therapeutic strategy.
Radionuclide MPI result and patient risk
Understanding post-MPI risk stratification begins with an understanding of the prognostic implications of a normal radionuclide MPI. An extensive literature exists documenting the low risk for adverse events after a normal radionuclide SPECT or PET MPI. A meta-analysis of almost 30,000 patients who were followed up after a normal stress SPECT revealed that the annual risk for myocardial infarction (MI) or cardiac death after a normal result was 0.5% (95% confidence interval, 0.3% to 0.7%), a finding also seen in other studies of pooled data. These results have been extended to outcomes after a normal PET study as well. , This low event rate—long associated with normal stress MPI—has been interpreted as implying that, in the absence of significant or limiting symptoms, patients with these findings can be managed conservatively. Importantly, the results of a normal MPI study differ considerably from a normal, zero score coronary calcium score. The former provides information regarding obstructive CAD, whereas the latter indicates the absence of atherosclerotic disease, a far earlier pathophysiologic stage with profoundly different implications.
Impact of clinical characteristics on risk after a normal stress radionuclide MPI
Although the risk for cardiac events is consistently lower in patients with normal SPECT MPI and PET MPI than in those with abnormal results, there are multiple subsets of patients in whom the risk associated with a normal, relative to an abnormal, MPI is low, but the absolute risk is not. For example, a study of 7376 patients with normal exercise or adenosine stress SPECT MPI—a cohort long considered low risk—who were followed for cardiac death or nonfatal MI examined the prognostic “warranty” of a normal MPI. Specifically, what is the temporal component of risk—that is, for how long after the initial study does risk remain low? Two important results emerged. First, not all normal studies were low risk, and specific patient characteristics identified in which patient subgroups this was the case. Second, the characteristics associated with increased risk and shortened time to a hard event (a warranty period of a normal MPI) included patient age, diabetes mellitus (DM; in particular, women with diabetes), known CAD, and the use of pharmacologic stress testing (the inability to exercise to a target heart rate) ( Fig. 17.1 ). Subsequent studies confirmed this concept , and the importance of DM and reduced left ventricular ejection fraction (LVEF) in this process. , Similarly, a meta-analysis supported the finding that event rates after a normal pharmacologic stress study are greater than those after a normal exercise stress MPI.

These studies support a paradigm that posttest patient risk is contextual by nature. As previously mentioned, the difficulty in defining thresholds of risk is that these definitions are inaccurate without consideration of the cohort to which they will be applied. No statement regarding absolute risk is valid in the absence of consideration of other available information. A discrepancy between observed risk after a normal MPI and the defined “low-risk” threshold has been frequently reported, particularly in studies examining elderly populations and those with diabetes. , , , A previous study examining post-SPECT risk in 5200 elderly patients revealed that annual cardiac mortality rates after normal MPI in this study were 1.0% in patients aged 75 to 84 years and 3.3% in those older than 85 years. Although these rates are greater than often reported after normal MPI, they are less than or equal to mortality rates for heart disease and major cardiovascular (CV) disease reported in the overall US population for individuals in these age groups ( Fig. 17.2 ). Furthermore, specific lower clinical risk groups have significantly lower mortality risk than the age-matched US population (e.g., exercise stress, normal resting electrocardiogram [ECG]). Conversely, studies have also shown that patients with prior CAD, abnormal resting ECG, and pharmacologic stress all had greater event rates after a normal MPI. Similar results were also observed in the setting of low exercise capacity, dyspnea, and multiple atherosclerotic risk factors. , These findings suggest that although MPI yields incremental value over clinical, historical, and demographic data, the converse is true as well. Estimation of risk after MPI must consider both the underlying risk of the population being examined and the MPI results.

These findings reported using SPECT MPI are generalizable to the results of PET MPI as well. Both single and multicenter studies with PET MPI have revealed normal MPI results to be associated with low risk for adverse events. , This finding has been confirmed in a meta-analysis. Interestingly, although the rates of cardiac death have been shown to be low after normal PET MPI, the rates of all-cause death, generally felt to be a more valid end point for observational studies, are greater in some studies. It is important to note that this is a reflection not of the modality (or of an inability of the modality to identify a low-risk cohort) but of the patient population referred to testing. With greater baseline risk and a high prevalence of significant noncardiac comorbidities, these patients are at greater risk for experiencing noncardiac deaths.
Risk for adverse events after abnormal MPI
Compared with event rates after normal MPI, the rates of adverse events after abnormal stress SPECT or PET MPI studies have been shown to be increased both in relative and absolute terms. The risk for adverse outcomes increased with worsening extent and severity of perfusion abnormality. It is this progressive increase of risk with worsening abnormalities that provides both incremental prognostic value over prior information and enhanced risk stratification. This finding has been found to be present irrespective of the end point used, the cohort examined, the type of stress used, the approach to MPI imaging, or the approach to interpreting perfusion abnormalities.
Importantly, at any level of MPI abnormality, patient risk varies with underlying patient risk. That is, clinical and historical patient information yields incremental value over MPI results ( Fig. 17.3 ). This pattern of graded risk extends to PET MPI as well. Indeed, the rates of cardiac death after stress PET MPI across categories of mild ischemia (5% to 10% of the left ventricle [LV]), moderate ischemia (10% to 20% of the LV), and severe ischemia (>20% of the LV) in a study of medically treated patients in a multicenter registry ( Fig. 17.4 A) suggest that increasing amounts of ischemia are associated with a stepwise increase in the risk for cardiac death. Second, the risk associated with any level of ischemia increases with an increase in underlying patient risk (e.g., increasing age, pharmacologic versus exercise stress). The increasing mortality rates across categories of stress perfusion defects have been reported with SPECT MPI as well. Third, there is an increase in unadjusted rates of cardiac death and all-cause death across the continuum of test results (i.e., normal and mildly, moderately, and severely abnormal; see Fig. 17.4 B). After risk adjustment, the hazard ratios for cardiac death (black numbers at the base of bars using normal PET MPI as comparator) across the abnormal MPI categories significantly increase with worsening test results. Interestingly, these hazard ratios do not increase as much with respect to all-cause death, probably because of the greater mortality rate in the normal MPI category. This pattern of results is similar to prior studies using SPECT MPI in a variety of patient subsets.


Ancillary markers of risk in radionuclide MPI
Although perfusion defects are the hallmark of identifying the presence and extent of CAD and the associated risk on radionuclide MPI studies, other related ancillary markers often contribute to risk assessment. Indeed, LV volumes, wall motion, and LVEF are now routinely obtained. Furthermore, with the increasing use of PET and its development, assessment of resting and peak stress myocardial blood flow (MBF) and the ratio of the two, myocardial flow reserve (MFR), are routinely measured and have been validated extensively (see Chapter 3 ). Additional markers of risk include the presence of transient ischemic dilation (TID), increased radiotracer uptake in the lungs, and increased right ventricular tracer uptake. A significant literature exists for most of these markers, indicating important prognostic value.
Left ventricular function and volumes
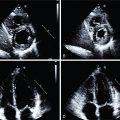
The routine acquisition of ECG-gated MPI allows for the quantification of regional and global systolic function and LV volumes. ECG-gated images are typically collected at rest and poststress (SPECT) or rest and during stress (PET). Historically, the powerful prognostic value of LVEF has been widely reported with MPI and other modalities, and gated SPECT-derived metrics have been shown to risk stratify and add incremental prognostic value. The latter was first described by Sharir et al., who examined poststress gated SPECT data in a cohort of 1680 patients, reporting that both LVEF and LV end-systolic volumes were powerful predictors of cardiac death and the composite end point of cardiac death or nonfatal MI, with significant relative risks for cardiac death in both categories of mild to moderate and severely abnormal stress perfusion subgroups. These results have been confirmed and extended by other groups with subsequent studies identifying gated SPECT and ischemia contributing incremental value to each other ( Fig. 17.5 ).

A drop in LVEF on the poststress images has also been shown to identify high-risk patients with multivessel CAD. Because stress PET–gated images reflect gated data at true peak stress, peak stress–gated data are more informative than poststress data (especially as the latter may be acquired at varying times poststress) and the difference between rest and peak stress carries more information and meaning. Indeed, a decrease or even a blunted increase in LVEF is associated with greater extent and severity of anatomic CAD and patient risk ( Fig. 17.6 ). , Conversely, normal LVEF, LV volumes, and LV wall motion identifies a cohort at lower risk irrespective of perfusion findings.

Myocardial blood flow and myocardial flow reserve
As described in Chapter 3 , a unique advantage associated with the use of PET is the opportunity to routinely capture accurate, validated, and reproducible measures of regional (vascular territory) and global MBF (in mL/min/g of tissue) at rest and during stress and estimate myocardial flow reserve (MFR). These quantitative measures capture the integrated effects of focal epicardial coronary stenoses, diffuse atherosclerosis, vessel remodeling, and microvascular dysfunction, thereby allowing for the assessment of flow perturbations at an earlier stage of the atherosclerotic process, as well as identifying patients with severe obstructive CAD. Importantly, although these metrics serve to identify hemodynamically significant epicardial coronary lesions that might be missed by balanced reduction in flow, they also extend the application of radionuclide MPI to the assessment of early preobstructive atherosclerotic disease, the identification of patients at risk, and the monitoring of disease progression/regression.
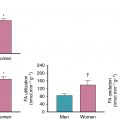
Murthy and colleagues reported the prognostic implications of MFR relative to indices of pre-MPI and PET myocardial perfusion in a cohort of 2783 patients referred for stress PET (median follow-up 1.4 years). Multivariable risk adjustment for clinical, historical, and PET data, including LVEF, change in LVEF with stress, summed stress score, and the use of early revascularization with cardiac death as an end point, revealed that, compared with the upper tertile of MFR, the hazard ratio associated with the middle tertile was 3.4 and the lowest tertile was 5.6. Risk stratification was achieved by MFR even after adjustment for the aforementioned considerable data. These results were extended to sex-related differences in patients without prior CAD and no evidence of PET-identified CAD-associated perfusion defects. More than half of men and women had abnormal MFR, and its presence was associated with adverse outcomes on risk-adjusted analyses. Importantly, these authors reported that 44% of men and 48% of women had normal stress perfusion and no discernable coronary artery calcium yet had abnormal MFR assessments, thus confirming MFRs potential to identify a significant number of patients with very early atherosclerosis, including those without disease by the most commonly used clinical tool for atherosclerosis. These findings were extended in cohorts with diabetes and chronic kidney disease (see Chapters 14 and 15 ). The presence of abnormal MFR in patients with diabetes but without CAD placed them at a level of risk similar to patients without diabetes with CAD, whereas patients with diabetes with a normal MFR had a level of risk not dissimilar to patients without CAD or without diabetes. Other subsequent studies have confirmed and extended these results. ,
Additional markers of risk
Transient ischemic dilation is among the oldest used markers from radionuclide MPI and refers to the appearance of a significantly larger LV cavity on the poststress compared with the resting images. A recent meta-analysis of the diagnostic and prognostic value of TID examined 31 studies and 9003 patients with prognostic follow-up. The authors reported that the presence of TID in the setting of an abnormal MPI was associated with an increased risk for adverse outcomes and an increased risk in high-risk patients with normal MPI. Nevertheless, there are some caveats with the assessment of TID that should be considered. Physiologically, transient LV dilatation as observed in radionuclide MPI reflects varying degrees of subendocardial hypoperfusion, which is commonly seen in high-risk patient groups, including those with diabetes mellitus, LV hypertrophy, abnormal scans consistent with multivessel CAD, and who are of an older age. This suggests that it may be a “fellow traveler” rather than a unique and independent marker of risk. , In addition, the TID ratio can be affected by patient (e.g., sex) and technical (e.g., radiotracer used) factors.
Increased right ventricular (RV) radiotracer uptake on SPECT MPI has been shown to be associated with severe CAD, particularly high-grade left main with less severe proximal right CAD. The mechanism of this finding is likely related to the appearance of increased counts in the RV due to relative LV hypoperfusion in the setting of normalization of image counts. Finally, an additional high-risk marker that has been reported is the presence of increased radiotracer uptake in the lung after stress imaging. This finding, shown to have incremental value over perfusion data alone, is believed to represent increased pulmonary capillary wedge pressure and can also be seen with other nonischemic etiologies of pulmonary capillary pressure (e.g., mitral regurgitation or stenosis).
Integrating information after radionuclide MPI
It is evident from this discussion that there are numerous prognostically relevant data elements available from a routine radionuclide MPI study. An important question that emerges is how to distill this information to formulate a global estimate of risk for a specific, individual patient. An approach that allows this is the use of large data sets to derive and validate weighted prognostic scores. The key advantages of this approach include the opportunity to consider numerous data elements and the potential to estimate patient risk in a very granular fashion.
Previous work has presented a prognostic score for patients undergoing vasodilator stress that incorporated clinical, historical, stress ECG results in addition to the perfusion data. The high mortality rate found in vasodilator stress patients permitted a robust model to be developed and a complex score generated. The score included age, diabetes, dyspnea as the presenting symptom, ECG findings, resting heart rate (HR), peak HR, percent myocardium ischemic, and percent myocardium fixed. Posttest treatment with revascularization versus medical therapy was also considered. The use of this score was shown to enhance the precision of risk estimation and stratification. This approach lends itself to incorporation into imaging software, thus permitting inclusion of the risk estimates in routine reporting. A future iteration of this approach lends itself to artificial intelligence to better weigh and estimate the relations between the numerous data elements available (see Chapter 32 ).
Post-MPI resource utilization and implications for risk assessment
Although risk stratification and prognostication dominate the radionuclide MPI outcomes literature, it is the examination of post-MPI resource utilization that permits insight into how this modality is used in practice and the appropriateness of this use. This assessment can be performed using several end points. Initially, studies examined early (within 60 or 90 days post-MPI) referral rates to catheterization and revascularization as a function of patient clinical information and MPI results. , , , Optimally, these studies examine cohorts of patients without prior CAD, thus obviating possible confounding by prior revascularizations and known coronary anatomy. Importantly, more recent studies have also included examination of early medical therapy (as an alternative and adjunct to catheterization referral) as well as the utilization of other noninvasive tests after MPI.
Several consistent patterns have emerged from these studies. First, the rates of referral to catheterization after MPI increase as a function of the MPI results—low referral rates after normal MPI, markedly increasing referral rates with worsening perfusion abnormalities—with MPI ischemia being the primary driver and other markers of ischemia also contributing (e.g., anginal presentation, ischemic ECG response, stress-induced chest pain; Fig. 17.7 ). , , , , , Interestingly, although fixed defects or scar have been shown to be associated with greater risk compared with ischemic defects, the rate of referral to catheterization in the setting of extensive nonreversible defects has been reported to be relatively low. Although the qualitative pattern of these results appear reasonable, it is the quantitative (absolute) values that raised questions. Both single and multicenter observational studies examining post-MPI referral patterns to catheterization in cohorts without prior CAD have reported that in the setting of moderate to severe amounts of ischemia, referral rates to catheterization did not exceed 40% to 60%. , , , A prospective multicenter registry enrolling patients without prior CAD referred to clinically order noninvasive imaging in 40 sites revealed that in patients with moderate to severe amounts of ischemia, the referral rates to 90-day catheterization after radionuclide MPI was in the range of 45% to 55% ( Fig. 17.8 ). This study, the first to examine medical therapy after testing, found that the results of testing did not influence posttest medical management. Indeed, only one in five patients in this study with severe test abnormalities were taking lipid-lowering agents, aspirin, and/or beta blockers at 90 days after testing. A similar pattern was observed in an ancillary analysis of the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial. Of note, subsequent studies revealed increased use of medical therapy after anatomic imaging with CCTA in both observational and clinical trial settings. These results suggest greater use of preventive measures with CCTA compared with functional imaging, as well as sex-related differences with undertreatment of women.