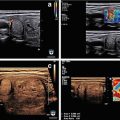
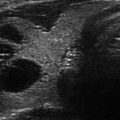
Figure 13.1
Ultrasound image of a three-fiber thyroid nodule laser ablation in a transverse US B-mode scan. Laser marks are seen as anechoic spots surrounded by hyperechoic rims. These are cavitation, due to tissue vaporization, and charring, respectively. The coagulation zone is hypoechoic parenchyma, clearly cleaved from viable tissue
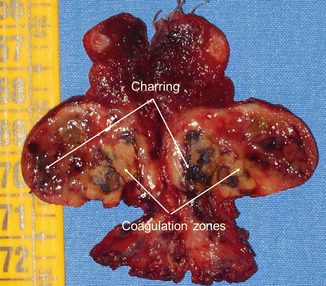
Figure 13.2
Macroscopic aspect of a benign nodule resected 1 month after LA treatment. Arrows show charring and coagulation zones
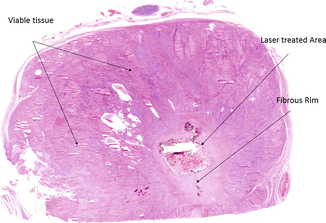
Figure 13.3
Microscopic changes (×5) occurring in a benign thyroid nodule resected after laser ablation. Arrows mark laser-treated area surrounded by a fibrous rim, within hyperplastic thyroid nodular viable tissue
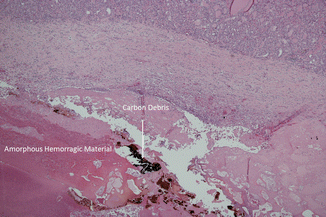
Figure 13.4
Nodule section at a further magnification (×20) shows amorphous hemorrhagic material with carbon debris (arrows) due to laser ablation
Charring is the main cause of decreased energy transmission, which in turn limits the coagulation zone. In addition, coagulation necrosis itself reduces optical penetration by about 20% in both normal and tumor tissue [4, 9]. Using a bare tip, almost spherical lesions with a maximum diameter of 12–16 mm can be produced. Lesion size can be increased by simultaneous use of multiple fibers in an array around the tumor, rather than repositioning a single fiber.
Laser Ablation in the Thyroid Gland
Outpatient ultrasound-guided (UG) interventional procedures have been proposed to treat benign solid thyroid nodules without open surgery. This approach is possible, thanks to the combined use of ultrasonography (US) and fine-needle aspiration biopsy (FNA) , which greatly reduces the need for diagnostic thyroidectomy [10–12]. The 2015 American Thyroid Association (ATA) management guidelines for patients with thyroid nodules and differentiated thyroid cancer suggest either no treatment or partial/total thyroid surgery for patients with benign solid thyroid nodular disease, depending on nodule size, growth, and symptoms [13], and the 2010 American Association of Clinical Endocrinologists—Associazione Medici Endocrinologi (Italian Association of Clinical Endocrinologists)—European Thyroid Association (AACE-AME-ETA) thyroid nodule guidelines have for the first time introduced UG interventional approaches as a possible choice for thyroid nodule clinical management [14]. In 2016 the AACE , American College of Endocrinology (ACE) and AME medical guidelines for clinical practice for the diagnosis and management of thyroid nodules confirmed the importance of UG minimally invasive procedures, namely, LA and radiofrequency ablation, for the treatment of benign, symptomatic, thyroid nodules [15]. The basic principle of locoregional treatment is to induce shrinkage of solid thyroid nodules using heat for tissue destruction. Thyroid LA was introduced by Pacella et al. in 2000 [16]. Since then several studies have been published on its effects on thyroid cold [17–29], cystic [30, 31], and hot nodules [32–38], including controlled trials [39–43], demonstrating that this technique is effective and safe. Indeed, we have been using LA in patients with benign thyroid cold nodules in Reggio Emilia, Italy, since 2002.
Technique
LA is an outpatient procedure carried out on fasting subjects. The flat tip technique is based on the insertion of a 300 μm plane-cut optic fiber through the sheath a 21G Chiba needle, exposing the bare fiber in direct contact with thyroid tissue for a length of 5 mm. In the thyroid gland, multiple fibers are inserted cranio-caudally one at a side of the other at 1.0 cm distance, in order to obtain an ellipsoid ablation that matches the ellipsoid shape of most thyroid nodules (Fig. 13.5). At variance with the square multiple fiber technique used for the liver, in the thyroid gland optimal geometrical configuration may be achieved inserting simultaneously the fibers (Fig. 13.6) in a triangle or in a line array depending on the nodule shape and size. With four fibers inserted simultaneously, combined with pullbacks, volume ablations up to 30 mL may be attained in a single session.
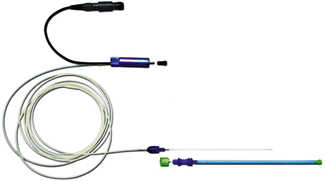

Figure 13.5
Three laser fibers inserted in Chiba 21-gauges needles along cranio-caudal major nodule axis; needles are separated by 1.0 cm

Figure 13.6
A laser fiber used for thyroid LA. (Photograph courtesy of ELESTA s.r.l. Calenzano, Italy)
The goal of LA procedure is to achieve the maximum ablation volume in a single outpatient session. The patient is placed on an operating table in the supine position with hyperextended neck. The operator, seated behind patient’s head, watches real-time US images. US equipment is used by an assistant sitting to the right of the patient. A nurse helps in maneuvers. Procedure is carried out in the dark; a scialytic lamp is used only for needle placement. Real-time imaging is the key support and it is used throughout all procedure steps. In Reggio Emilia, in the years 2002–2008, we used an Nd:YAG laser with 1064 nm near-infrared wavelength emission, equipped with a four-source beam splitter (DEKA M.E.L.A., Florence, Italy). In 2009, we substituted this with equipment composed of an ultrasound device and a laser unit (EcholaserX4®, Elesta, Florence, Italy). The EcholaserX4® (Fig. 13.7) permits the operator to use up to four laser sources, each with its own energy emission setting and independent activation. This helps matching the ablation zone to nodule size and shape. Contrast enhancement ultrasound (CEUS) study can be performed before and after LA in order to estimate ablation volume. Contrast media (Sonovue®, sulfur exafluoride microbubbles) is rapidly injected intravenously. Conscious sedation is obtained with i.v. midazolam (2–5 mg) in fractionated boli. Emergency care drugs and equipment, including a defibrillator, are in the interventional suite. Although an anesthesiologist is not present during LA sessions, we recommend performing LA in a healthcare facility where an anesthesiologist is available on call. Local anesthesia with 2% ropivacaine HCl subcutaneous and pericapsular infiltration (2–5 mL) is performed under US assistance with a thin (27 gauge) needle. Chiba 21-gauge needles (1–4) are placed manually along the longitudinal, cranio-caudal, major nodule axis at a distance of 10 mm, matching the anatomy of nodules as closely as possible. Fibers are inserted and laser is immediately turned on. An initial energy of 1200–1800 J/fiber with a mean output power of 3 W (range 2–4 W) is delivered starting 10 mm from the bottom of the lesion. A highly echogenic area due to tissue heating and vaporization gradually increases over time until coalescence between fibers is observed. Multiplanar US images on axial and longitudinal scans are performed by the assistant/sonographer throughout laser illumination, allowing real-time visual control of each source. By upward needle/fiber pullbacks of 10 mm, additional doses of laser energy are administered at each step until a distance of 5 mm from the cranial portion of the nodule is reached (Fig. 13.8). While small thyroid nodules may be ablated using a single optic fiber, large nodules up to 40–50 mm in width, 30–35 mm in thickness, and 50–70 mm in length (i.e., up to 30–60 mL) may be treated by combining multiple fiber placement, needle/fiber pullback, and high energies. The number of fibers, number of pullbacks, and total energy delivered are tailored to nodule volume. The duration of laser illuminations ranges from 6 to 30 min, depending on nodule size. Light irradiation is continuous; it is shortly suspended for fiber repositioning only in the event of pain, cough, or other side effects.
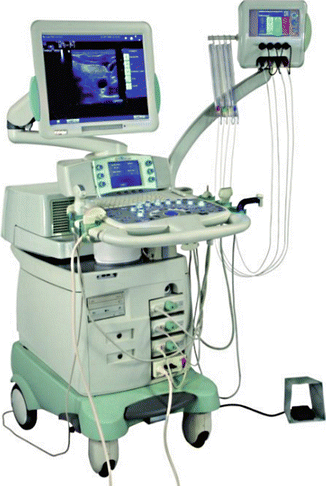
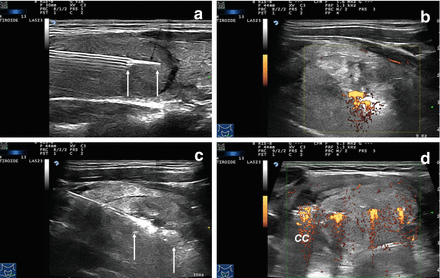

Figure 13.7
The Echolaser X4® (Elesta, Florence, Italy) equipment, composed of an ultrasound device and a laser unit. The small monitor is the touch screen laser display, permitting the operator to use up to four sources each with its own activation and energy emission setting. (Photograph courtesy of ELESTA s.r.l., Calenzano, Italy)

Figure 13.8
Multiplanar ultrasound imaging of a LA procedure with three fibers in a thyroid nodule. (a) Fiber exposed out of the tip of the needle (arrows). (b) Laser firing, color-Doppler imaging, longitudinal scan. (c) Laser firing, B-mode, longitudinal scan, first pullback. Arrows indicate the pullback area. (d) Laser firing, transverse scan, color-Doppler images during laser illumination showing the three needles at the same time. CC common carotid artery
Laser Ablation Post-procedural Care
Immediately after the LA procedure , all patients receive prednisone 20 mg i.v. bolus. An ice pack with mild pressure is applied on the neck. Patients are then taken to the recovery room, where they receive ketoprofen 100 mg or paracetamol 1 g and are kept under observation for about 2 h. Before leaving hospital, all patients undergo US examination. The day after the LA procedure, patients are started on a tapering oral methylprednisolone therapy of 16 mg daily for 5 days, 8 mg daily for 4 days, and 4 mg daily for 3 days. Oral pump inhibitors are simultaneously administered (lansoprazole 30 mg) for the 12 days of oral prednisone therapy.
Side Effects
Few complications and side effects have been reported in the published data [23, 28, 43, 44]. In our own large clinical experience, no patient has ever required emergency care or emergency surgery. Intra-operatory pain is usually absent or minimal. Should it occur, laser should be turned off, and fibers repositioned in a more central area of the nodule. Post-operatory pain may occur in 8–40% of patients, requiring additional medication. Intranodular bleeding during needle placement is controlled by rapid fiber insertion and laser illumination and should not prevent regular ablation procedure from being completed. In our hands, infrequent (less than 2.5%) complications were thyroid pericapsular bleeding; vagal symptoms with bradycardia; cough; reversible voice change (complete recovery in 1–2 months after an additional corticosteroid course); tumor rupture with subfascial effusion, disappeared in 3–4 months, with no permanent consequences; cutaneous burn; transient stridor; and hyper- or hypothyroidism.
A single case of tracheal laceration 50 days after LA has been reported in the literature. The patient underwent total thyroidectomy and tracheal repair [45].
Clinical Results in Benign Cold Thyroid Nodules
Nodule shrinkage in the available literature ranges from 36 to 82% of initial volume [17–43]. In our center, we reported safety and effects of Nd:YAG LA treatment in patients with benign nonfunctioning thyroid nodules in a 3-year follow-up [23]. In 2012 we presented data on a 5-year follow-up in 72 patients (51 females, 21 males, age 52.2 ± 12.3 years) treated with LA for nonfunctioning thyroid nodules [46]. Energy delivered was (mean ± SD) 8842 ± 6086 J with an output power of 3.1 ± 0.5 W. Five years after LA, mean ± SD nodule volume decreased from 28.1 ± 29.3 to 14.5 ± 17.6 mL, with a percent reduction of −49.6%. Volume reduction was related with good clinical response, i.e., cosmetic improvement and compressive symptoms reduction.
According to internal content, nodules were classified as follows: (a) compact (solid, iso-/hypoechoic, homogeneous, ≤10% of cystic content), (b) mixed (solid, inhomogeneous, with a 20–50% cystic content), and (c) spongiform (aggregation of multiple microcystic components in more than 50% of the nodule). Cystic nodules (fluid content ≥50%) were excluded from this series. In 25 spongiform nodules, volume decreased from 24.8 ± 25.9 to 7.7 ± 7.5 mL, with a reduction of −58.7%. In 14 mixed nodules, volume decreased from 41.1 ± 47.3 to 16.1 ± 17.4 mL, with a reduction of −48.3%. In 33 solid nodules, volume decreased from 26.4 ± 24.5 to 17.9 ± 22.0 mL, with a reduction of −26.8%. Hence, LA was more effective in patients with spongiform thyroid nodules as compared to mixed (P ≤ 0.01) and compact (P ≤ 0.001) nodules. Our data demonstrate that LA procedure was more effective and durable in patients with spongiform nodules . These results were confirmed by a study of Negro et al. published in 2016 [29]. The patients with spongiform nodules are therefore the best candidates for percutaneous LA procedure (Fig. 13.9).
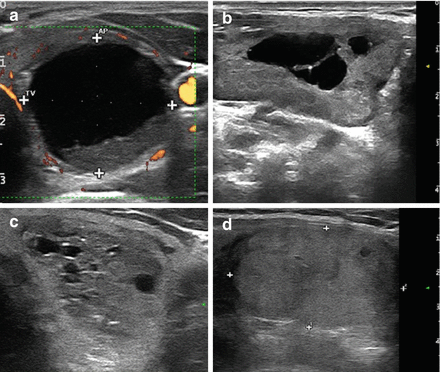

Figure 13.9
(a) Cystic thyroid nodule. (b) Mixed thyroid nodule. (c) Spongiform thyroid nodule. (d) Solid thyroid nodule
Clinical Results in Autonomously Functioning Thyroid Nodules
In a few case series studies, LA was found effective in treating patients with autonomously functioning thyroid nodules (AFTNs) in terms of hyperthyroidism control [32, 33, 35]. Nonetheless, other studies demonstrated that LA achieved only partial results in normalizing thyroid hormone levels in patients with AFTNs , and more treatment sessions were required [16, 34, 36, 41]. A combined treatment with LA and 131-iodine has been proposed for large toxic nodular goiters [37, 38]. In conclusion, the routine use of LA is not recommended for the routine treatment of AFTNs, but laser therapy may be considered when surgery or radioiodine are contraindicated or declined.
Clinical Results in Thyroid Cysts
Ethanol ablation (EA) is the first-line treatment for recurrent, benign, symptomatic, cystic (cystic portion >90%) or predominantly cystic (cystic portion less than 90% and greater than 50%) thyroid nodules [13–15, 47]. However, aspiration combined with LA is also effective in treating thyroid cysts [30, 31], albeit more expensive than EA. There are no studies comparing EA to LA for the treatment of thyroid cystic nodules. In our center we use EA for pure thyroid cysts and LA for multilocular thyroid cysts or predominantly cystic thyroid nodules since heat can destroy cystic septa and solid nodular tissue (Fig. 13.10).
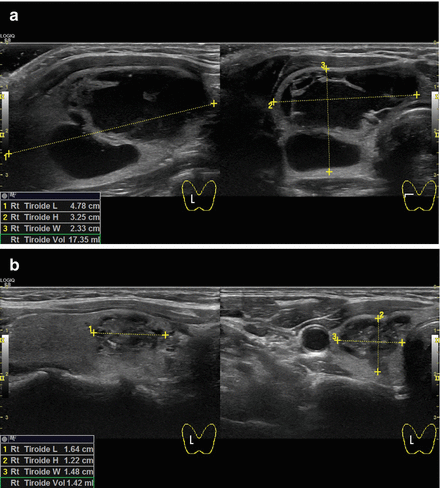

Figure 13.10
B-mode ultrasound images of a right complex multilocular thyroid cyst before (a) and 3 months after (b) aspiration and laser ablation in the same session. The thyroid nodule shrunk from 4.78 cm L × 2.33 cm H × 3.25 cm W (volume of 17.35 mL) to 1.64 cm L × 1.22 cm H × 1.48 cm W (volume of 1.42 mL) with a volume reduction of 91.8%
Clinical Results in Recurrent Thyroid Cancer
Occasionally well-differentiated thyroid cancer (DTC) may recur, mainly in the thyroid bed or in lymph nodes [48]. In this case, surgical removal and radioiodine is recommended [13]. However, repeated neck dissection is difficult due to distortion of normal tissue planes by scar tissue formation, and such operations are associated with a higher rate of complications such as recurrent laryngeal nerve injury, hypoparathyroidism, and skin scar formation [49]. In 2013 two case series studies reported the efficacy of LA for the local control of neck recurrences from papillary thyroid cancer (PTC) [50, 51]. Ablated metastatic lymph nodes shrunk, and serum thyroglobulin (Tg) levels declined significantly. Recently, two other studies confirmed the potential role of LA for the treatment of cervical recurrences from PTC [52, 53]. It seems that LA may be used in high-risk surgical patients or in patients refusing additional surgery as an alternative to repeated surgical resection of metastatic disease of PTC.
Laser Ablation in Primary Thyroid Papillary Microcarcinoma
Papillary thyroid microcarcinoma (PTMC) is usually associated with an excellent outcome. For patients with PTMC without extrathyroidal extension, and without clinical evidence of any lymph node metastases, the initial surgical procedure can be either a total thyroidectomy or a thyroid lobectomy [13]. However, management of PTMC may become a dilemma because of patients’ reluctance to have surgery or due to comorbidities. In 2013 we published a study with the aim to evaluate the clinical feasibility of LA on PTMC as a primary treatment and to prove histologically the absence of residual viable tumor after the LA procedure [54]. Three patients diagnosed by fine-needle aspiration cytology as positive for malignancy consistent with papillary thyroid carcinoma (category Thy 6 according to the Bethesda fine-needle aspiration cytology classification system) [55] were enrolled in the study after a full explanation of the protocol. These patients had positive fine-needle aspiration cytology for papillary thyroid cancer with US evidence of only one nodule 10 mm or less in greatest dimension and no other evidence of tumor in the thyroid gland or neck. All patients underwent percutaneous LA of the PTMC in the operating room under general anesthesia immediately before surgical removal of the thyroid gland.
After LA completion, the surgeon directly started a standard total thyroidectomy. Subsequent to surgery, the thyroid glands were submitted for histological evaluation. Tumor and surrounding parenchymal cellular vitality analysis were evaluated by immunohistochemistry on selected paraffin-embedded blocks using both thyroid transcription factor-1 (TTF-1) and antihuman mitochondria antibodies (clone 113-I). TTF-1 positivity was defined by nuclear labeling, while antihuman mitochondria antibody reactivity corresponded to a granular cytoplasmic staining.
The histological features were comparable in all three cases; that is, the neoplastic tissue around the cavitation showed typical changes of thermal damage, including cell shape distortion or cell shrinkage and nuclear chromatin condensation. On higher-power view, cases 2 and 3 were found to have incidental papillary microfoci far from the ablated zone. In case 2, a micrometastasis was observed in 1 perithyroidal lymph node out of 3 resected lymph nodes. There was no continuity between intrathyroidal PTMC foci and the lymph node metastasis.
The signs of thermal damage included a 2–3 mm rim of normal tissue around the tumor that faded away at the border of the cavitation. In the residual thyroid tissue, the follicular architecture was maintained, and there was no extrafollicular colloid spillage or hemorrhage. No vascular invasion was seen. In all cases, there was complete loss of TTF-1 and antimitochondria antibody staining in the whole ablated area and in the rim of normal tissue surrounding the tumor. In contrast, the immunoreactivity for TTF-1 and antimitochondrial antibodies was easily detectable in nontargeted tissue. This demonstrates that the ablated neoplastic tissue was irreversibly damaged.
In conclusion, this study proves that percutaneous LA is technically feasible for complete PTMC destruction. Now, LA may be useful in selected patients with PTMC, either when the surgeon or a patient refuses surgery or when the patient is at high surgical risk. However, LA cannot be recommended as a routine primary choice of treatment for PTMC since recognition of micro-multifocality and lymph node micrometastasis is not possible at present.
Clinical Results in Functional Parathyroid Adenomas
In 2012 we published a study assessing the long-term efficacy of LA in the treatment of primary hyperparathyroidism (PHPT) due to functional parathyroid adenomas (PAs) in six patients [56]. Two months after laser ablation, serum parathyroid hormone (PTH) and calcium levels decreased in six and five patients, respectively. At the last follow-up examination, i.e., 1–7 years after LA, serum PTH and calcium levels were above the normal range in six and three patients, respectively. Three patients underwent surgery for persistent PHPT. LA therapy was safe and without permanent side effects, albeit one patient reported transient dysphonia. In conclusion, we found that LA produces a transient reduction of serum PTH and calcium levels in patients with PAs, but it is not a lasting solution of hyperparathyroidism.
Radiofrequency Ablation in the Thyroid Gland
The goal of radiofrequency ablation (RFA) is to induce thermal injury to the tissue through electromagnetic energy deposition. Passage of alternating high-frequency current, between 200 and 1200 kHz through tissue, leads to a rise in temperature, without muscle contraction or pain [11]. The alternating electric field created in the tissue displaces molecules first in one direction, then in the opposite direction. Such agitation creates frictional heat. In the monopolar mode , which is the one most commonly used, the patient is part of a closed-loop circuit that includes a radiofrequency generator, an electrode needle, and a large dispersive electrode (ground pads) (Fig. 13.11). The discrepancy between the small surface area of the needle electrode and the large area of the ground pads causes the heat generated to be concentrated around the needle electrode inserted into target area. A typical treatment produces temperatures of 90 °C or more, resulting in coagulation necrosis within a few minutes, tissue desiccation, and consequent rise in impedance [57]. Small vessels are completely destroyed, and large vessels up to 3 mm in diameter are thrombosed. As for the laser, overheating with carbonization would limit heat transmission and tissue destruction. Instead, internally cooled tip needles maintain probe tip temperatures at around 90 °C without tissue charring. This improves the ability of the radiofrequency applicator to cause tissue ablation.

Figure 13.11
Thyroid RFA system. The patient is part of a closed-loop circuit that includes a radiofrequency generator, an electrode needle, and a large dispersive electrode (ground pads). The discrepancy between the small surface area of the needle electrode and the large area of the ground pads causes the heat generated to be concentrated around the needle electrode inserted into target area. (Illustration courtesy of STARmed Co., Ltd., Gyeonggi-do, Korea)
Devices
For monopolar thyroid RFA straight, internally cooled, short (7 or 10 cm), thin (18 or 19 gauge) electrode needles connected to a radiofrequency generator are currently used (Fig. 13.12). The active tips of the electrode needles may have a different length (0.5–2.0 cm) according to the desired ablation area [58, 59]. Experience with bipolar radiofrequency electrode needles for thyroid ablation is still limited [60–62]. In the past other types of electrode needles (e.g., multi-tined expandable electrodes) had been used for thyroid RFA, but their use is no longer recommended [63–65].


Figure 13.12
The VIVA RF Generator® (STARmed, Korea) creates predictable and consistent ablation zones by using optimized ablation modes for various nodules. It also has a smart user interface to provide the exact information related to procedure details, and it records patient treatment data. The star RF electrodes Fixed ® (STARmed, Korea) are used in percutaneous thyroid radiofrequency ablation procedures. The coolant circulation system of the electrode needle maintains the suitable impedance at the surface of the electrode. It also prevents tissue adjacent to the active tip from charring and helps to make a spherical ablation zone. (Photograph courtesy of STARmed Co., Ltd., Gyeonggi-do, Korea)
Technique
Thyroid RFA is performed as an outpatient procedure in an interventional suite. Patients should be fasting. The patient is placed on an operation bed in the supine position with hyperextended neck, and a venous catheter is inserted in a forearm vein. The operator is seated behind the patient’s head and controls the US monitor throughout the procedure (Fig. 13.13). A multiparametric monitor is connected to the patient displaying a continuous single lead electrocardiogram, pulse oximetry, blood pressure, and respiratory rate. Conscious sedation is obtained with intravenous (i.v.) midazolam (2–5 mg) in fractionated boli in order to decrease the patient’s anxiety, swallowing, cough, and movements. Local anesthesia with 2% HCl ropivacaine subcutaneous and pericapsular infiltration (2–5 mL) is performed under US assistance with thin (27 gauge) needles (Fig. 13.14).
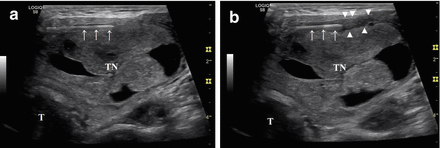

Figure 13.13
During thyroid RFA the operator is seated behind the patient’s head and controls the US monitor throughout the procedure. An assistant (nurse, sonographer, or 2nd operator) is helpful during the thyroid ablation

Figure 13.14
Local anesthesia with 2% ropivacaine HCl pericapsular infiltration (2–5 mL) before RFA. (a) A 27-gauge spinal needle (arrows) is inserted into the pericapsular space above the anterior surface of the thyroid nodule (TN). (b) The anesthetic liquid (arrowheads), which appears hypoechoic in US imaging, accumulates around the needle (arrows). B-mode, longitudinal, US image of a large, predominantly solid, right thyroid nodule. T: trachea
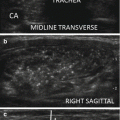
In the RFA monopolar technique, the patient is part of a closed-loop circuit: ground pads (dispersive electrodes), applied to both thighs, are connected to a radiofrequency generator, and the generator is connected to an electrode needle. These electrode needles are straight, internally cooled, 7 cm, and 18 gauge and have active tips of usually 1.0 cm for most thyroid nodules. The ablation is performed according to the “moving shot” technique: the nodule is divided into multiple conceptual areas, and thereafter the nodule is ablated unit by unit by moving the electrode tip (Fig. 13.15) [66–68]. The ablation starts from the deepest portion of the nodule with a trans-isthmic approach (Fig. 13.16). The needle is inserted transisthmically at the higher level according to patient anatomy. This insertion allows the repositioning of the needle as much as 60–90 degrees with respect to the entry point in order to achieve ablation of more medially located tissue. This maneuver does not increase side effects, particularly vocal cord palsy, because while continuously tilting the probe, careful surveillance of the area medial to the needle is required to detect US signs of heating (gas microbubbles) when the needle is inserted close to the tracheoesophageal groove where the inferior laryngeal nerve runs (the so-called danger triangle). When a hyperechoic area appears and when impedance increases, the tip is moved backward to an untreated more superficial area. The maneuver is repeated with repositioning the needle, until all areas are ablated. Usually RF power starts with 30 W, and 5 W upward adjustments are made up to 60 W. Higher RFA power is used in Korea [69]. The successful ablation of a unit is confirmed by the appearance of a hyperechoic area—due to microbubbles—and the abrupt increase of impedance (the so-called break point) registered on the RF generator monitor.
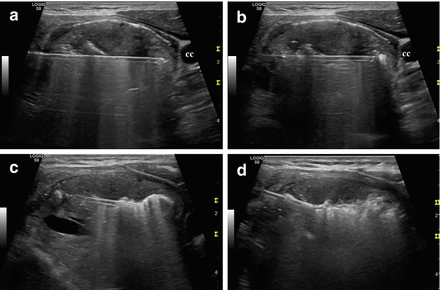
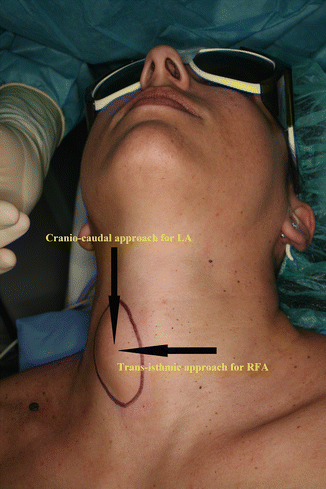

Figure 13.15
Longitudinal ultrasound images of the “moving shot” thyroid RFA technique. The needle is inserted transisthmically. The thyroid nodule is divided into multiple imaginary ablation units, and RFA is performed unit by unit. (a) Insertion of the electrode needle toward the common carotid (cc) artery. (b–d) The needle is moved within the thyroid mass by pulling it back and tilting it upward from deep to superficial layers of the nodule. The ablated area appears hyperechoic because of tissue vaporization

Figure 13.16
Approaches for thyroid LA and RFA. Cranio-caudal (longitudinal) approach, through long axis of the thyroid nodule, is used for LA. Trans-isthmic approach, via short axis of thyroid nodule (from medial to lateral aspect), is used for RFA
To treat mixed nodules, the cystic fluid is first aspirated and then the ablation is performed.
Radiofrequency Ablation Post-procedural Care
The procedural care after the RFA is the same as for the LA (see above).
Side Effects
Complications during thyroid RFA are uncommon, but they should be taken into account. A large multicenter Korean study reported an overall complication rate of 3.3% associated with the RFA treatment of benign thyroid nodules [70]. In order to prevent complications, it is important to have a thorough knowledge of sonographic anatomy of the neck [71].
Intraoperative pain is usually well controlled by means of local anesthesia with ropivacaine and conscious sedation with midazolam [68]. Notwithstanding, in case of intense pain, local or radiating to the jaw, teeth, chest, or back, the RF generator is turned off. Subsequently, the electrode needle is repositioned in a more central area of the nodule and the ablation can proceed. Intranodular bleeding during needle insertion may occur and is seen as a rapidly expanding hypo/anechoic signal within nodular tissue. It can be stopped by swift needle-electrode insertion and heat administration. Intranodular bleeding does not prevent ablation procedure. Thyroid pericapsular bleeding is seen as a hypoechoic layer surrounding the thyroid. Pericapsular hematomas can be controlled by compression of the neck for a few minutes. Neck bruising may follow a few days later, and it disappears in about 3–4 weeks. A rarely reported complication is vasovagal reaction which presents with bradycardia, hypotension, vomiting, and defecation. If this reaction is observed, the bed is tilted in Trendelenburg position, and maneuvers are temporarily interrupted until spontaneous recovery, which occurs in a few minutes. This possible event is due to vagus nerve stimulation as the nodule may displace the common carotid artery and the internal jugular vein. For this reason, knowledge of cervical vagus nerve localization is essential in thyroid RFA [72]. Cough during thermal ablation is due to trachea stimulation, and the tip of the needle should be pulled back.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree