Authors
Year
Study type
Patients (n)
MR field strength
Laser source
Treatment method
Real-time imaging
Atri et al.
2009
Patient
1
NA
830 nm (Indigo diode)
Transperineal
CEUS
Lindner et al.
2009
Patient
12a
NA
830 (Indigo diode)
Transperineal
CEUS
Lindner et al.
2010
Patient
4
NA
980 nm (Visualase diode)
Transperineal
CEUS
Raz et al.
2010
Patient
2
1.5 T
980 nm (Visualase diode)
Transperineal
MRI + CEUS
Woodrum et al.
2010
Human cadaver
5
3 T
980 nm (Visualase diode)
Transperineal
MRI
Woodrum et al.
2011
Patient
1
3 T
980 nm (Visualase diode)
Transperineal
MRI
Oto et al.
2013
Patient
9
1.5 T
980 nm (Visualase diode)
Transperineal
MRI
In 2009, Atri et al. [32] introduced the application of CEUS-guided LITT. They treated a patient with biopsy-proven, solitary focus, low-risk PCa, visualized on mpMRI prior to the procedure. During and after photothermal therapy treatment, they observed large hypocontrast regions surrounding the treatment fibers, indicating the presence of an avascular lesion. The lesion they measured using CEUS also corresponded to tissue devascularization seen on a gadolinium (Gd)-enhanced MRI lesion 1 week after the procedure.
One of the first reported phase 1 studies assessing the feasibility of using a CEUS-guided focal laser with MRI was by Lindner et al. in 2009 [37]. They looked at 12 patients, including a patient from the 2009 Atri et al. study [32], with biopsy-proven low-risk PCa (T1c or T2a, PSA <10 ng/ml, Gleason score ≤6, only 1 of 12 cores <30 % cancer following transrectal ultrasound (TRUS)-guided biopsy) that underwent interstitial photothermal ablation. MRI was used to confirm and target the lesion, and a 3D ultrasound was fused to this to help guide the laser. Out of this cohort, 3 patients complained of perineal discomfort, 2 developed mild hematuria, 2 had hematospermia, and 1 complained of fatigue. There was no reported decrease in mean urinary or sexual function scores at 1, 3, and 6 months postoperatively. TRUS-guided 10-core systematic prostate biopsies performed 3–6 months after ablation showed 6 patients (50 %) had completely negative biopsies, 2 patients had a tumor found on the contralateral untreated side, and 4 patients had a residual tumor in previously treated areas. Of these 4 patients, 2 were found to have minimal disease on follow-up biopsy, and 2 were found to have cores greater than 50 % of Gleason 6 disease. This same group also performed a study of 4 patients who underwent LITT followed by RP in 2010 [38]. They correlated MRI-calculated ablated volume to the volume of homogenous necrosis seen on whole-mount histology. Their results showed there was a very good correlation (r = 0.89) suggesting that post-ablation MRI could be a useful tool in determining the extent of actual tissue ablation.
One of the initial experiences in using real-time MRI guidance during LITT was performed by Raz et al. in 2010 [25]. MRI guidance allowed for improved visualization of the target, more accurate direction of the laser fiber, real-time monitoring of the ablation site and surrounding tissue, as well as immediate feedback on the extent of the treatment. The patients from this study were discharged without complications, and preservation of the neurovascular bundles was noted. There were also no adverse effects noted after 1 month of treatment.
Woodrum et al. in 2010 [39] also initially explored the feasibility of performing transperineal LITT for focal targets in the prostate using real-time MRI guidance. Previous studies had been focused on 1.5 T field strengths, but this study demonstrated the practicality of a 3.0 T MRI, which offered greater spatial and temporal resolution. They were able to verify that a perineal guidance grid allowed for accurate needle placement through a transperineal route at the appropriate depth in the prostate. Furthermore, they were able to correlate the gross appearance of the ablation zone to the temperature mapping estimates.
In 2011, Woodrum et al. [40] explored the use of 3.0 T MR focal laser thermal ablation therapy within the prostate bed in PCa recurrence. They reported a study in which they used LITT with real-time MR temperature mapping using the PRF shift to treat a patient with a documented recurrence after RP. The case was successful in treating the patient’s recurrence with a decrease in PSA from 2.0 to 0.42 ng/ml at 2 months after therapy. Early follow-up showed no posttreatment incontinence, rectal wall injury, or other complications.
In 2013, Oto et al. [41] published the results of their phase 1 trial (federally registered NCT01192438) evaluating the safety and feasibility of MRI-guided focal laser therapy in men with clinically low-risk PCa (clinical stage T1c–T2a, PSA <10 ng/ml, Gleason ≤7, minimum of 12 biopsy cores with 3 or fewer containing cancer, no single biopsy with >50 % tumor involvement, and a suspicious lesion visible on MRI corresponding to the biopsy site). In total, 9 patients were treated with 8 having Gleason grade 6 cancer and 1 having grade 7 cancer. No major complications or serious adverse events after ablation occurred. MR imaging-guided biopsy of the ablation site was performed at 6 months follow-up. This revealed benign prostate in 7 patients and Gleason grade 6 cancer in 2. A retrospective review of the ablation images were performed showing that the lesion site was not completely covered by the ablation zone for the 2 patients with positive findings at follow-up biopsy. Quality-of-life (QOL) surveys were recorded at baseline and at 1, 3, and 6 months post-ablation. The study reported no statistically significant change from baseline in sexual function or urinary symptom assessments.
Lindner et al. also updated their phase 1 study to 38 men in 2013 continuing to show minimal complications after a year of posttreatment follow-up. A concerning outcome in this study was that 26 % of men treated showed a positive biopsy outside the ablated region at 4 months follow-up. This finding stresses the need for larger multi-institutional studies with longer follow-up to further elucidate the efficacy and side effect profile of focal therapies [42].
LITT Experience at the National Institutes of Health
The National Cancer Institute (NCI) at the National Institutes of Health (NIH) in Bethesda, Maryland, USA, is currently the site of an institutional review board-approved, phase 1 LITT trial [43]. The selection criteria include patients with organ-confined clinical T2a Gleason ≤7 (3 + 4) in 4 cores or less that also have been screened with mpMRI using a 3.0 T MRI scanner (Achieva, Philips Healthcare, Best, the Netherlands). Standardized MRI acquisition is composed of T2-weighted, axial DWI, 3D spectroscopy, and axial DCE sequences using a combination of a 16-channel cardiac surface coil (SENSE, Philips Healthcare) placed over the pelvis together with an endorectal coil (BPX-30, Medrad, Pittsburgh, Pennsylvania) filled with PFC-770. Suspicious lesions are independently evaluated and graded on a validated scoring system [27] incorporating the number of positive functional MRI modalities by two experienced genitourinary radiologists. Targeted biopsies are then performed using an MR/US fusion platform along with standard 12-core random TRUS biopsy. Within 6 months, biopsy-proven cancerous lesions are treated using the FDA-approved Visualase 980 nm diode laser system (Visualase Inc., Houston, Texas, USA).
The ablation procedure is performed in gantry, using a 3.0 T magnet to acquire an mpMRI image to help locate the desired tumor, guide laser fiber placement, and confirm optimal placement. A transperineal approach is utilized which reduces the risk of rectal wall damage with the advantage of improved access to the apical and anterior portions of the prostate. An MR-compatible transperineal template is fixed to an endorectal coil, and an MR image of the prostate is taken for grid registration and to ensure proper placement of the fiber before treatment. Titanium trocars and guide catheters are used to ensure proper depth of applicator placement confirmed by the planning software (Visualase Inc., Houston, Texas, USA). When the laser tip is confirmed to be in the desired location, the laser is activated at a power level insufficient to cause thermal injury so that appropriate placement of the applicator and proper operation of thermal imaging can be verified. A single ablation typically lasts 1–2 minutes with repeated imaging following cessation of laser activity to ensure cooling of the prostate. Real-time assessment of ablative zones is achieved using MR thermometry (Fig. 11.1).
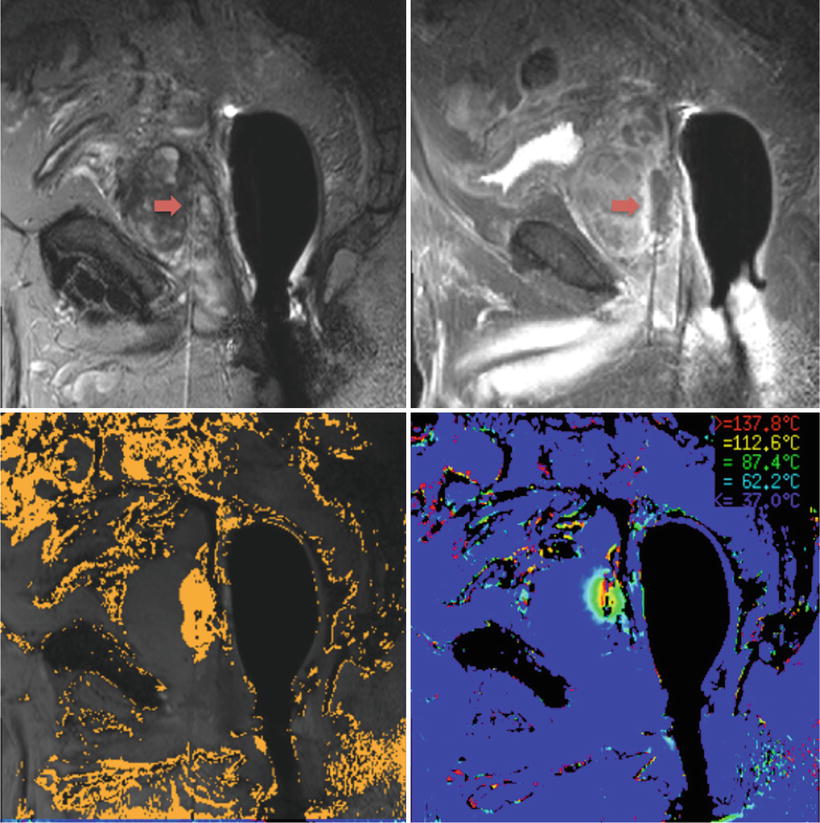

Fig. 11.1
MRI images obtained before (top left) and after (top right) LITT on a patient at the NIH. The tip of the needle can be visualized by the red arrow. Destruction mapping is seen on the bottom left and real-time thermometry is seen on the bottom right
Increasing laser power output, larger caliber fibers, and new lasers have allowed for larger ablation zones, which often help to achieve better tumor-free margins and decrease local tumor progression but risk unwanted collateral damage to nearby structures [18]. The use of hydrodissection has been incorporated in LITT at the NIH to facilitate tumors abutting the rectum, creating a buffer to protect vital structures. Although normal saline and 5 % dextrose are commonly used, migration of fluid is difficult to control and may require repeat instillation of fluid during the procedure. This had led to the design of a thermoreversible poloxamer solution that can be injected as a solution but forms a semisolid gel at room temperature [44]. Studies have shown favorable outcomes especially during percutaneous microwave ablation [45]. The NIH is currently working on using this versatile solution in hydrodissection during LITT.
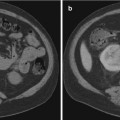
Patients undergoing LITT at the NIH undergo scheduled mpMRI to monitor treatment outcomes (Fig. 11.2). Routine mpMRI is taken 1 day, 6 months, and 1 year after treatment with annual imaging taken subsequently. The accrual ceiling for the phase 1 study is now complete with a phase 2 study under way.
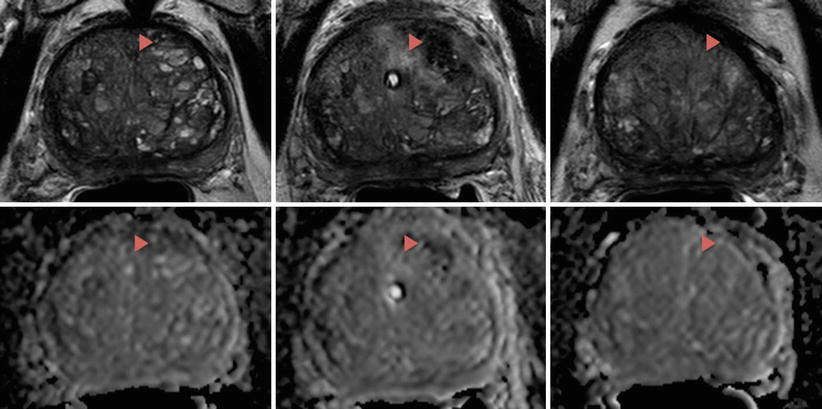

Fig. 11.2
T2 (top row) and DWI (bottom row) images of a patient who underwent LITT at the NIH. The patient had clinical T1 stage, Gleason 7 (3 + 4) disease involving the left mid-base anterior central gland with a pretreatment PSA of 6.5. The lesion, ablation zone, and scar tissue can be visualized by the red arrows before the ablative procedure (left column), 1 day after the ablation (middle column), and 1 year after the ablation (right column), respectively
Conclusion
Focal therapy is an evolving treatment paradigm that still requires further investigation. The application of focal treatment for PCa has been met with many difficulties: criteria for patient selection, precise localization, visualization and characterization of clinically significant cancer foci, accurate and precise guidance of ablative energy in the treatment zone, oncologic efficacy evaluation, and surveillance modalities [20]. Despite these challenges, preliminary phase 1 studies have shown that the LITT is feasible with good short-term efficacy and safety. Current uncertainties regarding long-term oncologic control will have to be answered as LITT moves out of its infancy and into larger clinical studies. The success of focal therapy revolves around the ability to accurately locate the tumor site as well as stage and risk-stratify patients, which has been progressing due to the recent advances in soft tissue imaging and mpMRI. Further advancements in using 3D MRI/US co-registration platforms will enable urologists to not only localize clinically significant disease more accurately but will serve as a tool to destroy MRI targets possibly under local anesthesia or in an office-based operating room [22]. It is likely that MRI-guided LITT will be adopted in the future as a powerful tool for urologists to treat prostate cancer.