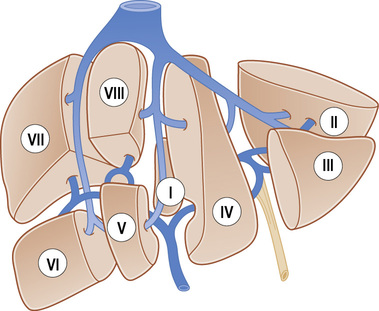
• The liver is subdivided anatomically into 8 segments in an anticlockwise fashion: • The caudate lobe (segment I): this is autonomous, receiving vessels from both the left and right portal vein branches and the hepatic artery • Blood supply to liver: ⅔ is from the portal vein • Venous drainage: this is via the 3 hepatic veins into the IVC (30% of patients have accessory draining veins) – usually an accessory inferior RHV draining of segments VI or VII • Aberrant gastric venous drainage of segments I and IV: this is correlated with focal fatty change within this segment • Gadolinium-based agents: these will generate enhancement (T1WI) • Hepatobiliary specific agents: the target includes the reticuloendothelial system or hepatocyte • This provides a global view of the liver and helps characterize a lesion if CT or MRI is not available • 99mTc-sulphur colloid or albumin colloid is usually used – 90% is taken up by the Kupffer cells (10% is taken up by the spleen) • FDG-PET has a relatively limited role (as normal liver takes up FDG) Acute or chronic liver inflammation • Infection: this is usually due to hepatitis • This represents the endpoint of a wide variety of chronic disease processes that cause hepatocellular necrosis and ultimately lead to hepatic fibrosis and nodular regeneration: • Early cirrhosis: there is increased reflectivity (due to fat infiltration and fibrosis) • Advanced cirrhosis: a nodular liver margin (this is seen especially with a high-frequency transducer and if ascites is present) • Increased hepatic arterial flow can be seen with advanced cirrhosis (due to a reduced portal venous contribution to the hepatic blood supply) • Increased flow in a large recanalized para-umbilical vein may ‘steal’ blood from the right portal vein branch – this can lead to reversed flow within the right portal vein but normal hepatopetal flow within the main and left portal veins • Early cirrhosis: there is uneven radionuclide uptake (and lobar morphological changes with progression) • Advanced cirrhosis: less sulphur colloid is taken up by the liver and increased extrahepatic activity is seen within the heart, and the reticuloendothelial cells of the bones and lungs • These are composed of vascular channels of varying size (cavernous to capillary) which are endothelium lined • It is the commonest benign hepatic tumour (it can be multiple in <10% of cases) • Capillary haemangioma: a well-defined lobular homogeneous hyperechoic lesion (large lesions can be heterogeneous) • Cavernous haemangioma: a hypoechoic lesion (due to the larger vascular channels) • T2WI: there is increasingly high SI with extended echo times (malignant lesions are typically less prominent with later echo times) • T1WI + Gad: centripetal enhancement from the periphery to the centre over a period of minutes 1. A well-circumscribed hepatic mass with peripheral, nodular and interrupted enhancement progressing centripetally to uniform enhancement (most common) 2. Immediate uniform enhancement (small capillary haemangiomas < 1.5cm). 3. Peripheral nodular enhancement with centripetal progression but persistent central hypointensity (giant haemangiomas > 5cm). A large haemangioma may sequester thrombocytes, leading to a thrombocytopenia Typical imaging features of common liver lesions FNH, focal nodular hyperplasia • T1WI: intermediate or minimal low SI • T2WI: intermediate to high SI • T1WI + Gad: marked, homogeneous arterial phase enhancement that becomes isointense during the portal venous phase • Other lesions with a central scar: hepatocellular adenoma (HCA) • Other lesions demonstrating Kupffer cell activity: HCA • MR imaging with a hepatocyte-specific contrast agent may help confirm the hepatocellular origin of the mass: • A rare benign hepatic tumour arising spontaneously or in association with glycogen storage disease type 1 (when it is often multiple) • It is a vascular lesion composed primarily of hepatocytes which have a tendency to accumulate fat and glycogen • It may demonstrate a fibrous pseudocapsule and a central scar (like FNH) An uncomplicated adenoma has similar appearances to a region of FNH • T1WI: a well-defined isointense or slightly high SI lesion • T2WI: variable signal intensity but are often mildly hyperintense relative to the liver • T1WI + Gad: heterogeneous or uniform marked enhancement (arterial phase), equilibrating with the liver parenchyma (portal phase) • DWI: variable signal intensity depending on the presence of blood or necrosis • Focal fat variation within the liver parenchyma is due to alterations in the underlying blood supply and venous drainage • Water and fat signal intensities will combine on the in-phase imaging, but cancel out on the out-of-phase imaging • Lesions containing significant amounts of fat will lose SI on the out-of-phase images (relative to the in-phase images) • Out-of-phase images: these can be identified as the intra-abdominal viscera are outlined by an ‘inky black’ line
Liver
ANATOMY AND IMAGING TECHNIQUES
ANATOMY
Couinaud classification
 The horizontal left and right portal veins separates the superior segments (II, IVa, VIII, VII) from the inferior segments (III, IVb, V, VI)
The horizontal left and right portal veins separates the superior segments (II, IVa, VIII, VII) from the inferior segments (III, IVb, V, VI)
 The three vertical hepatic vein branches further subdivide the segments:
The three vertical hepatic vein branches further subdivide the segments:
 it has an independent venous drainage directly into the IVC
it has an independent venous drainage directly into the IVC
Vascular anatomy
 ⅓ is from the hepatic artery
⅓ is from the hepatic artery
LIVER IMAGING TECHNIQUES
Contrast agents
 Iron oxide particles: these are superparamagnetic causing susceptibility induced proton dephasing (with a reduced SI) within normal tissues on T1WI and particularly T2WI
Iron oxide particles: these are superparamagnetic causing susceptibility induced proton dephasing (with a reduced SI) within normal tissues on T1WI and particularly T2WI  larger particles (50–100nm) are taken up by the Kupffer and endothelial cells and are rapidly cleared from the circulation
larger particles (50–100nm) are taken up by the Kupffer and endothelial cells and are rapidly cleared from the circulation  smaller particles are retained within the circulation for a longer period providing a prolonged ‘intravascular’ phase of enhancement (and therefore providing an angiographic effect as a blood pool agent)
smaller particles are retained within the circulation for a longer period providing a prolonged ‘intravascular’ phase of enhancement (and therefore providing an angiographic effect as a blood pool agent)
 Hepatocyte-specific paramagnetic agents (e.g. Mn-DPDP [mangafodipir trisodium])
Hepatocyte-specific paramagnetic agents (e.g. Mn-DPDP [mangafodipir trisodium])  these accumulate within hepatocytes, and then undergo biliary excretion
these accumulate within hepatocytes, and then undergo biliary excretion  they will cause enhancement of normal liver parenchyma and the biliary tree (T1WI) – a low SI indicates an abnormal area
they will cause enhancement of normal liver parenchyma and the biliary tree (T1WI) – a low SI indicates an abnormal area
Liver scintigraphy
 99mTc-labelled red blood cells can be used if a haemangioma is suspected
99mTc-labelled red blood cells can be used if a haemangioma is suspected
BENIGN DIFFUSE LIVER DISEASE
HEPATITIS
Definition
 Hepatitis A: this is usually benign and self-limiting
Hepatitis A: this is usually benign and self-limiting
 Hepatitis B: this can present as an asymptomatic carrier state, or with acute or chronic hepatitis, fulminant hepatic failure, and hepatocellular carcinoma
Hepatitis B: this can present as an asymptomatic carrier state, or with acute or chronic hepatitis, fulminant hepatic failure, and hepatocellular carcinoma
 Hepatitis C: an acute or chronic hepatitis with possible subsequent cirrhosis
Hepatitis C: an acute or chronic hepatitis with possible subsequent cirrhosis
CIRRHOSIS
CIRRHOSIS
DEFINITION
RADIOLOGICAL FEATURES
US
 a coarse heterogeneous echotexture
a coarse heterogeneous echotexture  hypoechoic regenerating nodules
hypoechoic regenerating nodules
 Attenuated hepatic veins can be seen with end-stage disease (due to the liver atrophy)
Attenuated hepatic veins can be seen with end-stage disease (due to the liver atrophy)
 Pure hepatic fibrosis increases liver reflectivity (resulting in loss of the portal vein branch margins) but will not significantly alter its attenuation – this can be used to discriminate fibrosis from fatty infiltration
Pure hepatic fibrosis increases liver reflectivity (resulting in loss of the portal vein branch margins) but will not significantly alter its attenuation – this can be used to discriminate fibrosis from fatty infiltration
Doppler US
Colloid scintigraphy
 ‘Colloid shift’: with the development of portal hypertension there is splenomegaly and a reduced activity halo around the liver
‘Colloid shift’: with the development of portal hypertension there is splenomegaly and a reduced activity halo around the liver
BENIGN SOLID LIVER LESIONS
BENIGN SOLID LIVER LESIONS
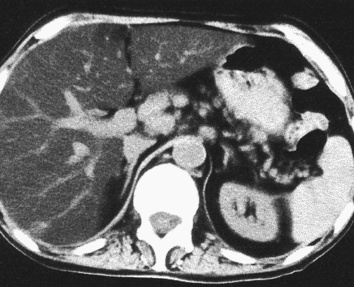
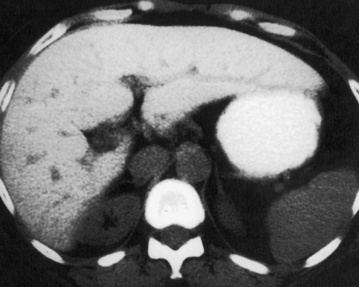
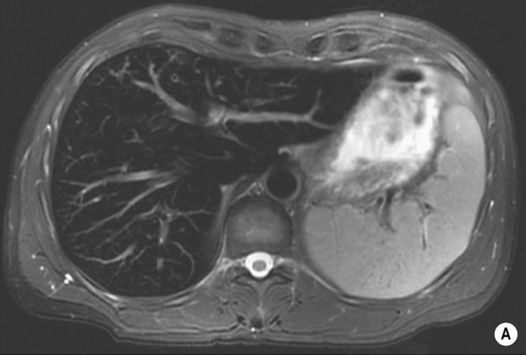
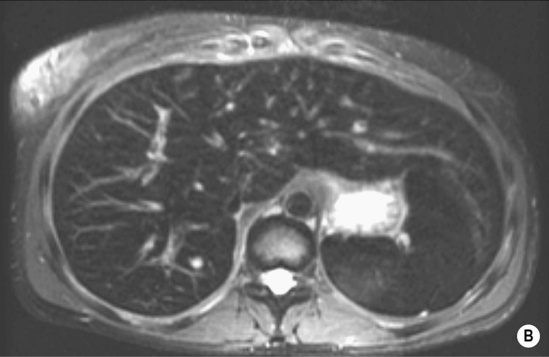
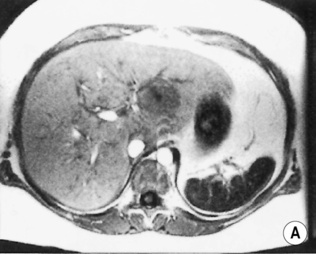
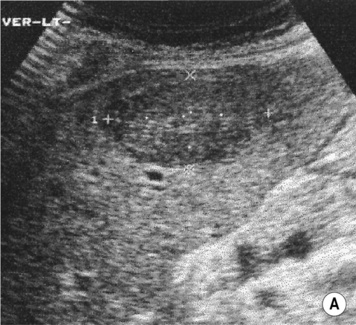
HAEMANGIOMA
Definition
 there is often intervening fibrous tissue of varying amounts
there is often intervening fibrous tissue of varying amounts
 Capillary haemangioma: the usual form
Capillary haemangioma: the usual form
 Cavernous haemangioma: this accounts for most neonatal and infantile haemangiomas (and some adult lesions)
Cavernous haemangioma: this accounts for most neonatal and infantile haemangiomas (and some adult lesions)
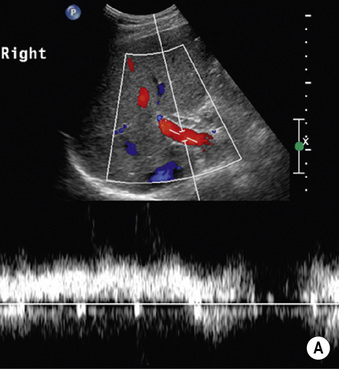
Radiological features
 there is no Doppler signal (due to the very slow vascular flow through the dilated channels)
there is no Doppler signal (due to the very slow vascular flow through the dilated channels)  it can appear very similar to some metastases (e.g. from a GI primary)
it can appear very similar to some metastases (e.g. from a GI primary)
 a Doppler signal is usually detectable (there are more rapid flow rates)
a Doppler signal is usually detectable (there are more rapid flow rates)
 ‘Lightbulb’ sign: homogeneously high SI (greater than that of the spleen and approaching that of cyst fluid)
‘Lightbulb’ sign: homogeneously high SI (greater than that of the spleen and approaching that of cyst fluid)
 there are three distinct enhancement patterns:
there are three distinct enhancement patterns:
 Small (< 1.5cm) lesions: these may fail to demonstrate characteristic T2WI signal changes (due to partial volume effects) or the typical enhancement pattern
Small (< 1.5cm) lesions: these may fail to demonstrate characteristic T2WI signal changes (due to partial volume effects) or the typical enhancement pattern
 Larger (> 4cm) lesions: these often have atypical internal features such as an area of central fibrosis that can prevent complete infilling during contrast enhancement
Larger (> 4cm) lesions: these often have atypical internal features such as an area of central fibrosis that can prevent complete infilling during contrast enhancement
Kasabach–Merritt syndrome
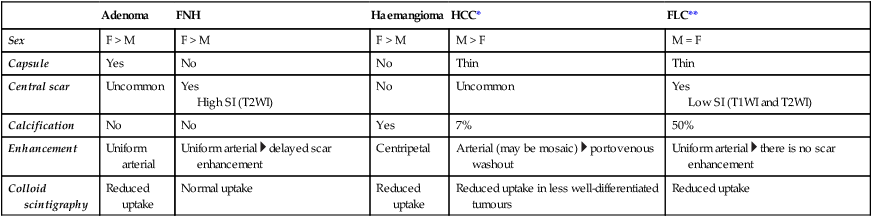
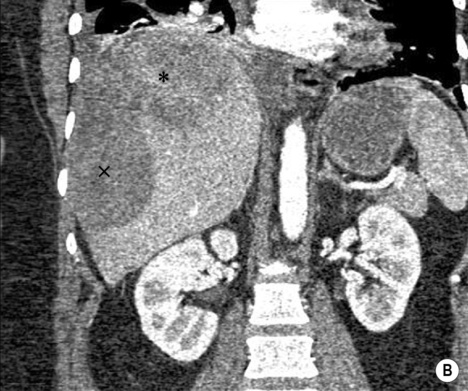
Adenoma
FNH
Haemangioma
HCC*
FLC**
Sex
F > M
F > M
F > M
M > F
M = F
Capsule
Yes
No
No
Thin
Thin
Central scar
Uncommon
Yes
High SI (T2WI)
No
Uncommon
Yes
Low SI (T1WI and T2WI)
Calcification
No
No
Yes
7%
50%
Enhancement
Uniform arterial
Uniform arterial  delayed scar enhancement
delayed scar enhancement
Centripetal
Arterial (may be mosaic)  portovenous washout
portovenous washout
Uniform arterial  there is no scar enhancement
there is no scar enhancement
Colloid scintigraphy
Reduced uptake
Normal uptake
Reduced uptake
Reduced uptake in less well-differentiated tumours
Reduced uptake
BENIGN SOLID LIVER LESIONS
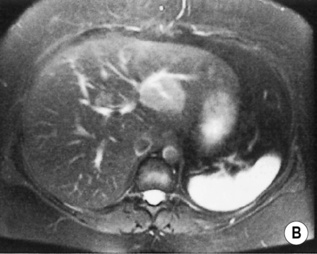
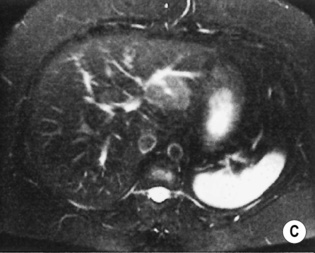
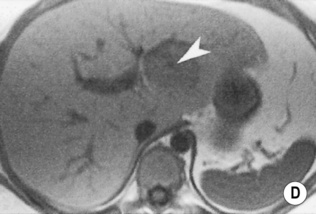
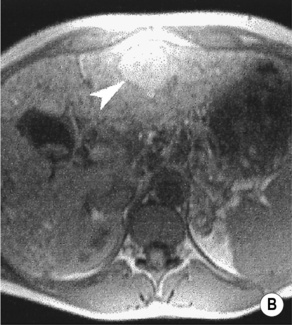
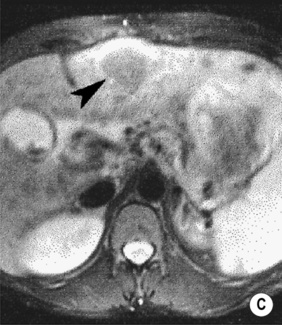
FOCAL NODULAR HYPERPLASIA (FNH)
MRI
 a low SI central scar
a low SI central scar
 a high SI central scar
a high SI central scar
 there can also be a peripheral, ring-type delayed enhancement pattern on delayed images obtained 1 h after hepatocyte selective gadolinium chelate administration
there can also be a peripheral, ring-type delayed enhancement pattern on delayed images obtained 1 h after hepatocyte selective gadolinium chelate administration
Pearls
 hepatocellular carcinoma
hepatocellular carcinoma  haemangioma
haemangioma
 a well-differentiated hepatocellular carcinoma
a well-differentiated hepatocellular carcinoma
BENIGN SOLID LIVER LESIONS
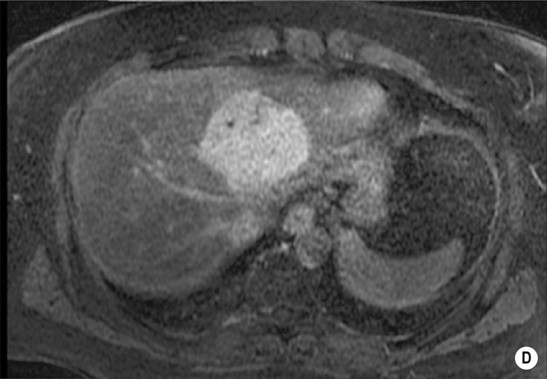
HEPATIC ADENOMA
Definition
 there are no portal tracts or bile ducts present
there are no portal tracts or bile ducts present  although Kupffer cells are absent, Kupffer cell activity can be seen in up to 20%
although Kupffer cells are absent, Kupffer cell activity can be seen in up to 20%
 there is a tendency to outgrow its blood supply, resulting in haemorrhage, thrombosis and necrosis
there is a tendency to outgrow its blood supply, resulting in haemorrhage, thrombosis and necrosis
Radiological features
 there can be hyperintense foci secondary to haemorrhage or intracellular fat
there can be hyperintense foci secondary to haemorrhage or intracellular fat
 haemorrhage or necrosis: this leads to a heterogeneous appearance
haemorrhage or necrosis: this leads to a heterogeneous appearance
 they are typically not as vascular as FNH
they are typically not as vascular as FNH
 They can demonstrate delayed contrast material washout with or without a delayed-enhancing pseudocapsule
They can demonstrate delayed contrast material washout with or without a delayed-enhancing pseudocapsule
 They are hypointense on 1-h to 3-h delayed images obtained with Gd-BOPTA
They are hypointense on 1-h to 3-h delayed images obtained with Gd-BOPTA
 Although venous washout is generally worrisome for malignancy, an adenoma may demonstrate venous washout (usually worrying for malignancy) – it is the only ‘benign’ hypervascular mass that may do so (cf. delayed isoenhancement with FNH)
Although venous washout is generally worrisome for malignancy, an adenoma may demonstrate venous washout (usually worrying for malignancy) – it is the only ‘benign’ hypervascular mass that may do so (cf. delayed isoenhancement with FNH)
BENIGN SOLID LIVER LESIONS
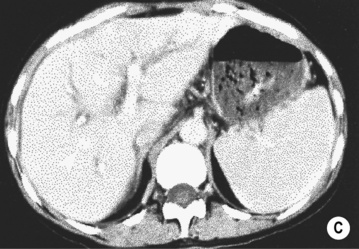
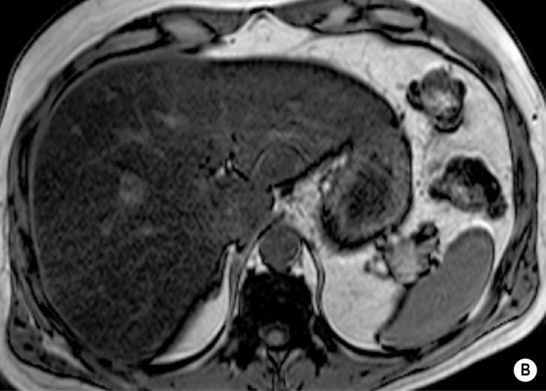
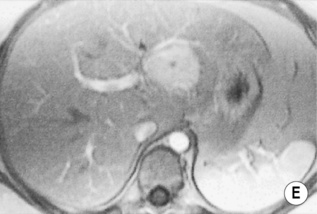
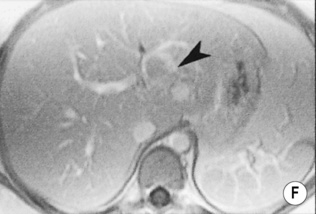
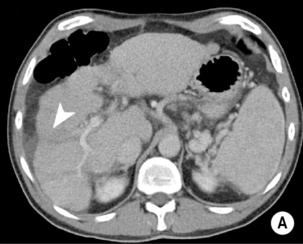
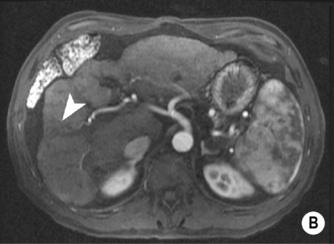
FOCAL FAT
DEFINITION
 it can cause diagnostic confusion with a tumour
it can cause diagnostic confusion with a tumour
RADIOLOGICAL FEATURES
MRI – ‘chemical shift’ or ‘in- and out-of-phase’ imaging
 as both image sets use a different TE, one needs to compare any signal change with a non-fat-containing organ (e.g. spleen) or correct for T2 signal changes using T2 mapping
as both image sets use a different TE, one needs to compare any signal change with a non-fat-containing organ (e.g. spleen) or correct for T2 signal changes using T2 mapping
 this occurs because at the organ–intra-abdominal fat interface the imaged voxel contains both fat and water and will therefore lose signal intensity (voxels located internally within the organ or intra-abdominal fat will tend to contain predominantly fat or water only and therefore not lose signal intensity)
this occurs because at the organ–intra-abdominal fat interface the imaged voxel contains both fat and water and will therefore lose signal intensity (voxels located internally within the organ or intra-abdominal fat will tend to contain predominantly fat or water only and therefore not lose signal intensity)
Liver

 there is a mean peak velocity of 15–25cm/s with slight respiratory variation
there is a mean peak velocity of 15–25cm/s with slight respiratory variation the liver usually has attenuation values of 54–60HU (8–10HU greater than the spleen)
the liver usually has attenuation values of 54–60HU (8–10HU greater than the spleen) they will therefore appear low attenuation on portovenous imaging.
they will therefore appear low attenuation on portovenous imaging.
 obesity
obesity  diabetes mellitus
diabetes mellitus  cystic fibrosis
cystic fibrosis  malnourishment
malnourishment  total parenteral nutrition
total parenteral nutrition  tetracyclines
tetracyclines  steroids
steroids  ileal bypass surgery
ileal bypass surgery the liver architecture is preserved
the liver architecture is preserved  there will be uniform enhancement post IV contrast medium administration
there will be uniform enhancement post IV contrast medium administration drugs (e.g. methotrexate)
drugs (e.g. methotrexate) gallbladder wall thickening
gallbladder wall thickening
 uptake occurs via the reticuloendothelial system (e.g. the Kupffer cells within the liver, bone marrow and spleen)
uptake occurs via the reticuloendothelial system (e.g. the Kupffer cells within the liver, bone marrow and spleen)
 previous amiodarone treatment and Thorotrast exposure may give similar appearances
previous amiodarone treatment and Thorotrast exposure may give similar appearances intracellular iron deposition exerts a local susceptibility effect leading to an abnormal reduction in liver SI
intracellular iron deposition exerts a local susceptibility effect leading to an abnormal reduction in liver SI  NB: normal hepatic parenchyma is brighter than adjacent skeletal muscle on T1W1 + T2W1
NB: normal hepatic parenchyma is brighter than adjacent skeletal muscle on T1W1 + T2W1 antenatal infections (e.g. CMV, rubella, enterovirus, toxoplasmosis, herpes simplex, and spirochaete)
antenatal infections (e.g. CMV, rubella, enterovirus, toxoplasmosis, herpes simplex, and spirochaete)  metabolic disorders (e.g. cystic fibrosis, α1-antitrypsin deficiency, tyrosinaemia and galactosaemia)
metabolic disorders (e.g. cystic fibrosis, α1-antitrypsin deficiency, tyrosinaemia and galactosaemia) a heterogeneous coarse liver parenchyma
a heterogeneous coarse liver parenchyma  a visible gallbladder (>1.5cm) without the triangular cord sign (cf. biliary atresia)
a visible gallbladder (>1.5cm) without the triangular cord sign (cf. biliary atresia) if cholestasis is severe, reduced extraction and excretion may make it difficult to distinguish this from biliary atresia
if cholestasis is severe, reduced extraction and excretion may make it difficult to distinguish this from biliary atresia this is hepatotoxic and triggers an inflammatory response that progresses to cirrhosis
this is hepatotoxic and triggers an inflammatory response that progresses to cirrhosis generalized cirrhotic changes
generalized cirrhotic changes T2WI: low SI
T2WI: low SI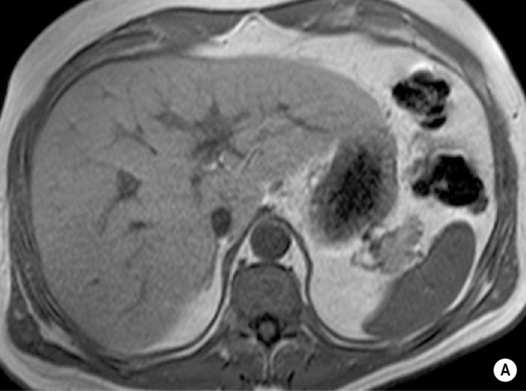

 hepatitis B
hepatitis B  storage disorders (e.g. haemochromatosis and Wilson’s disease)
storage disorders (e.g. haemochromatosis and Wilson’s disease)  biliary cirrhosis
biliary cirrhosis  certain drugs
certain drugs reduced main portal venous blood flow (<10cms-1 mean peak) or hepatofugal portal venous flow
reduced main portal venous blood flow (<10cms-1 mean peak) or hepatofugal portal venous flow  collateral vessel development (e.g. left gastric, splenorenal, paraoesophageal, or retroperitoneal collaterals) including a recanalized para-umbilical vein
collateral vessel development (e.g. left gastric, splenorenal, paraoesophageal, or retroperitoneal collaterals) including a recanalized para-umbilical vein lobar atrophy or hypertrophy
lobar atrophy or hypertrophy  ascites
ascites  portal vein thrombosis
portal vein thrombosis
 it can assess portal vein patency, flow direction and bulk flow volume
it can assess portal vein patency, flow direction and bulk flow volume portal hypertension (± variceal bleeding)
portal hypertension (± variceal bleeding)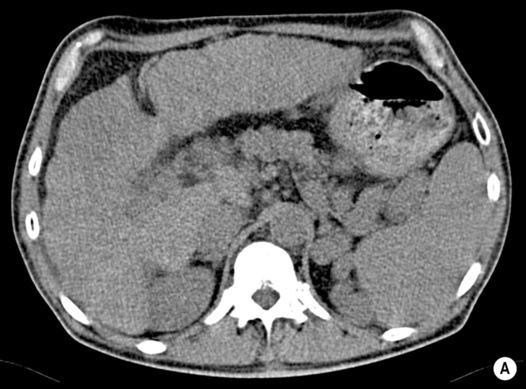
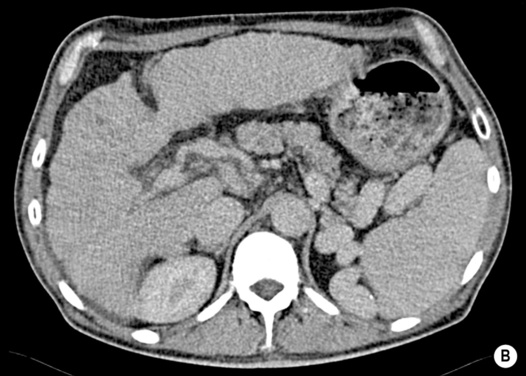
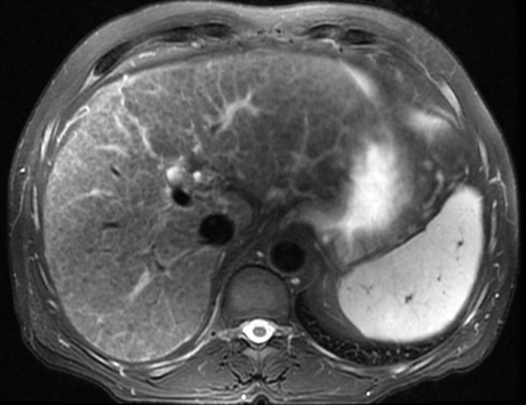
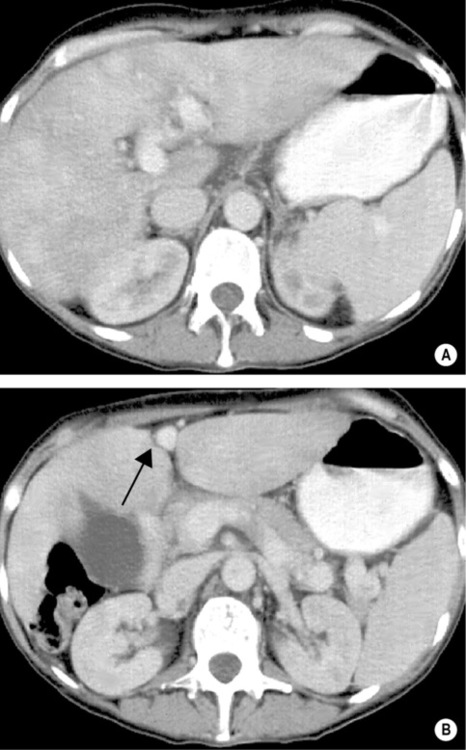
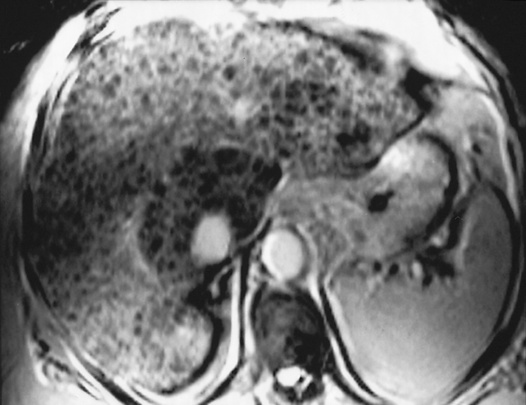
 larger lesions may rarely cause discomfort or undergo spontaneous rupture (F>M)
larger lesions may rarely cause discomfort or undergo spontaneous rupture (F>M) thrombi, calcification, fibrosis and scarring are variably present
thrombi, calcification, fibrosis and scarring are variably present characteristic imaging features are demonstrated if a lesion is between 2 and 4cm in size.
characteristic imaging features are demonstrated if a lesion is between 2 and 4cm in size.
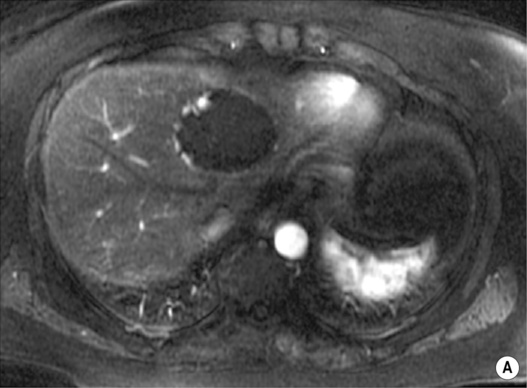
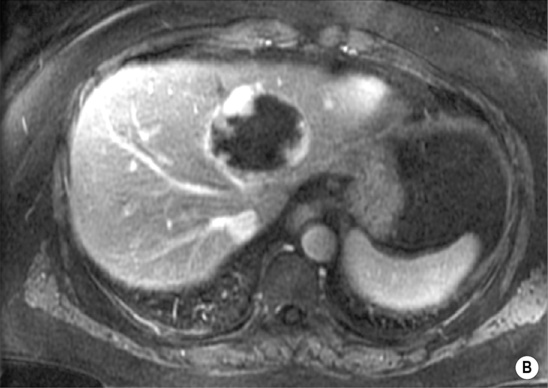
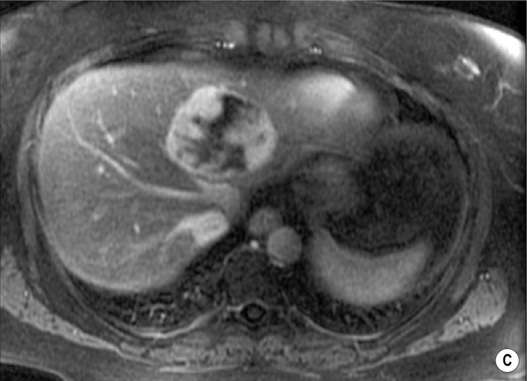
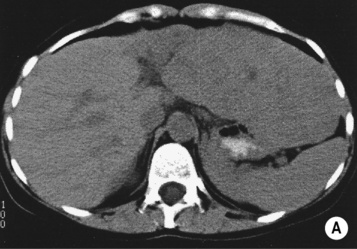
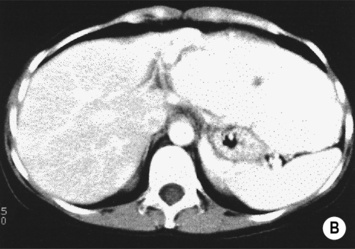
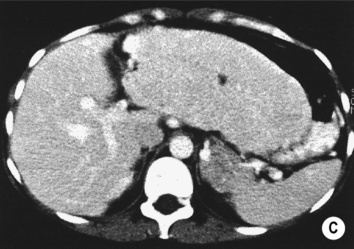
 however, there is a lack of normal liver architecture (e.g. there are absent portal tracts)
however, there is a lack of normal liver architecture (e.g. there are absent portal tracts)
 there is no true capsule
there is no true capsule  calcification, necrosis and haemorrhage are extremely rare (even large lesions do not usually outgrow their blood supply)
calcification, necrosis and haemorrhage are extremely rare (even large lesions do not usually outgrow their blood supply) the central scar is rarely seen
the central scar is rarely seen the lesion demonstrates the same attenuation as the surrounding liver
the lesion demonstrates the same attenuation as the surrounding liver  there is a central low attenuation scar
there is a central low attenuation scar there can be large peripheral feeding vessels
there can be large peripheral feeding vessels the specificity increases with iron oxide agents (which are taken up by the Kupffer cells)
the specificity increases with iron oxide agents (which are taken up by the Kupffer cells)
 radiating vessels spread out to supply the lesion
radiating vessels spread out to supply the lesion

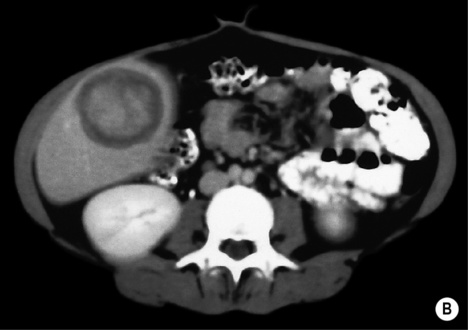
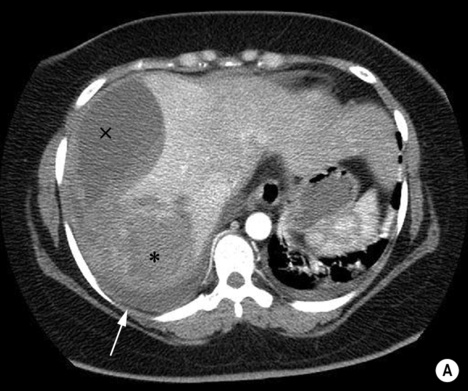
 androgenic steroids
androgenic steroids if rupture occurs there can be pain or life-threatening haemorrhage
if rupture occurs there can be pain or life-threatening haemorrhage it will be of similar attenuation to normal liver (or lower attenuation if there is a substantial fat component)
it will be of similar attenuation to normal liver (or lower attenuation if there is a substantial fat component)  intraparenchymal haemorrhage will appear as high attenuation thrombus on unenhanced images (which may extend through the capsule and into the peritoneum)
intraparenchymal haemorrhage will appear as high attenuation thrombus on unenhanced images (which may extend through the capsule and into the peritoneum)
 there is a risk of liver dysfunction, haemorrhage and HCC formation
there is a risk of liver dysfunction, haemorrhage and HCC formation some regenerative nodules may appear more prominent than others, causing diagnostic confusion with a HCC
some regenerative nodules may appear more prominent than others, causing diagnostic confusion with a HCC low SI if there is accumulation of iron (‘siderotic nodules’)
low SI if there is accumulation of iron (‘siderotic nodules’) these nodules then fade to isointensity (differentiating from HCC)
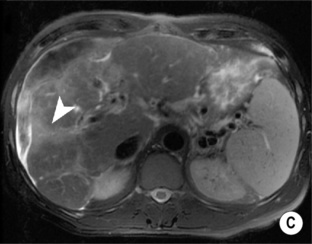
these nodules then fade to isointensity (differentiating from HCC)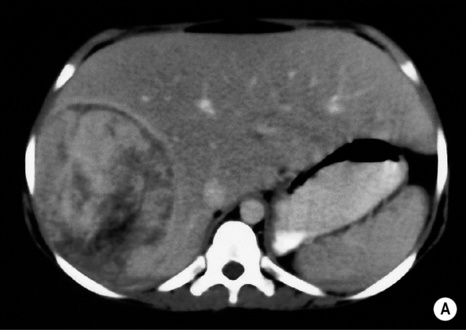

 the cranial aspect of the gallbladder fossa
the cranial aspect of the gallbladder fossa  the posterior aspect of segment IV
the posterior aspect of segment IV fat and water protons have different resonant frequencies – over time these will alternatively be in and out of phase with each other
fat and water protons have different resonant frequencies – over time these will alternatively be in and out of phase with each other  imaging at specific predetermined times will give either in- or out-of-phase images (they are out of phase 2.2 ms after an excitation pulse and in phase 4.4 ms after excitation)
imaging at specific predetermined times will give either in- or out-of-phase images (they are out of phase 2.2 ms after an excitation pulse and in phase 4.4 ms after excitation) diagnosis usually requires biopsy
diagnosis usually requires biopsy they will enhance (but remain low attenuation on unenhanced and portal phases)
they will enhance (but remain low attenuation on unenhanced and portal phases) T2WI: there is a characteristic appearance of multiple high SI lesions
T2WI: there is a characteristic appearance of multiple high SI lesions