31
Liver and Spleen
The use of angiography and venography for the diagnosis and staging of liver and splenic lesions has been nearly eliminated in recent years as a result of the advent of sonography, computed tomography (CT), and magnetic resonance imaging (MRI); however, interventional radiology procedures are being used more frequently for the treatment of these disorders. Advances in catheter, guide-wire, stent, and embolization technology resulted in the development of innovative percutaneous procedures for the management of tumors, trauma, and portal hypertension.
 Liver
Liver
Liver tumors
The two most frequent types of primary malignant liver tumors are hepatocellular carcinoma and cholangiocarcinoma. Metastases are the most common malignant neoplasms affecting the liver. Most patients with multiple liver metastases are not candidates for surgical resection and have a poor prognosis. Patients with metastases from neuroendocrine tumors have a better prognosis but may experience severe symptoms related to excessive hormone production.
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC, or hepatoma) is the most common primary liver malignancy, with most cases occurring in Asia and southern Africa.1 More than one million patients die of HCC per year worldwide. The prognosis of HCC without treatment is poor, with a median survival of 1.6 months reported in the Asian population.2 The development of HCC is associated with a history of hepatitis B or hepatitis C infection and cirrhosis. Seventy-five percent of hepatomas occur in cirrhotic livers. Ninety percent of patients with hepatomas are hepatitis B or C virus antigen carriers.1
Frequently, HCC is discovered as an incidental finding in patients with cirrhosis and portal hypertension. Symptoms include hepatomegaly, abdominal pain, fever, weight loss, ascites, and jaundice. HCC produces alpha-fetoprotein, which is elevated in the serum of about 70% of patients.3 Alpha-fetoprotein level can be used for diagnosis as well as a measure of response to therapy.
Sonography, CT, and MRI are commonly used imaging modalities for the screening, diagnosis, and staging of HCC.4 HCC may be solitary, multicentric, or diffuse. Angiographic features that may be found in HCC include enlarged feeding arteries, neovascularity, irregular tumor stain, arteriovenous (AV) shunting, and portal vein invasion (Fig. 31-1). Hepatic vein invasion may be identified during the parenchymal phase of a hepatic arteriogram or an inferior venacavogram. The arteriographic findings of hypervascular metastases or hemangiomas may appear similar on arteriography. Therefore, arteriography frequently lacks specificity for the diagnosis of HCC.
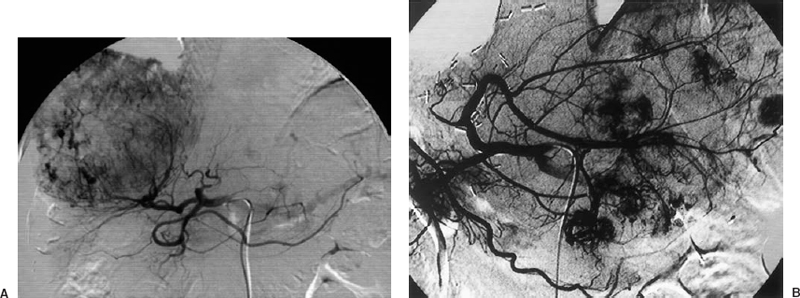
FIGURE 31-1. A: Solitary. B: Multicentric hepatocellular carcinomas.
Portal hypertension that is newly diagnosed or worsening may be the first clinical sign of HCC. HCC may invade the portal venous system and lead to portal hypertension. During angiography, invasion of the portal vein by HCC may be identified as a filling defect within the portal vein or thrombosis of the portal vein (Fig. 31-2). The tumor thrombus may contain vascular “threads and streaks.”5 The portal vein normally is not opacified on hepatic artery injection; however, arteriovenous shunting is often present in cases of HCC. This high-output communication between the hepatic artery and the portal vein may cause reversal of portal venous flow and portal hypertension.
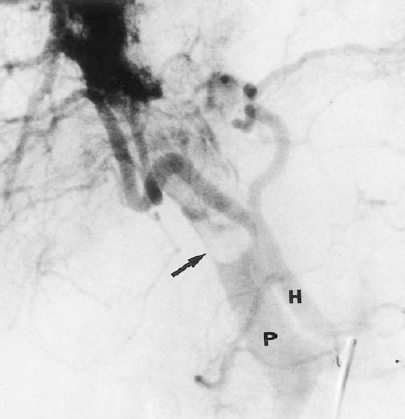
FIGURE 31-2. Hepatocellular carcinoma. Hepatic artery injection demonstrates arteriovenous shunting and invasion of the portal vein (arrow). H, hepatic artery; P, portal vein.
Surgical resection and liver transplantation are the treatment methods most likely to result in cure; however, they are not options in patients with diffuse disease. At initial diagnosis, many patients already have satellite nodules, extrahepatic metastases, or vascular invasion that may preclude surgical therapy. The median survival is 4 to 6 months for patients with unresectable tumors. Systemic chemotherapy has not been shown to be effective.6
Several percutaneous techniques have been described for the treatment of HCC and unresectable metastatic liver disease. These include percutaneous injection of ethanol, acetic acid, or hot saline into the tumor and thermal ablation with radiofrequency, laser, microwaves, or freezing (cryoablation). Therapy can be delivered through a catheter into the hepatic artery, including chemotherapy infusion, hepatic artery embolization, or hepatic artery chemoembolization.7,13 The rationale for therapy via the hepatic artery is that the blood supply to hepatic tumors is typically from the hepatic artery, not from the portal vein.
Chemoembolization is a combination of intraarterial infusion of a chemotherapeutic agent and the introduction of an embolizing agent for occlusion of the tumor vascular supply. Chemoembolization decreases blood flow to the tumor, resulting in ischemia and extending the time of contact of the chemotherapeutic drug with the tumor.14 Embolization materials such as polyvinyl alcohol (PVA), gelfoam, and iodized oil (Ethiodol) have been used in combination with chemotherapeutic agents.15 One study described the use of hepatic artery embolization with PVA particles without any chemotherapeutic agent.1 No treatment method has proven to be superior.
Chemoembolization can be technically challenging, with a great potential for complications. Preembolization arteriography of the celiac, superior mesenteric, and hepatic arteries is performed to define the blood supply of the tumors, to identify variant arterial anatomy, and to assess the patency of portal vein and the direction of portal venous flow. The presence of portal vein occlusion is a poor prognostic factor that increases the risk of liver necrosis following chemoembolization. Other contraindications to chemoembolization are severe liver failure, tumor involvement of greater than 70% of the liver, and biliary obstruction.6
Complications of chemoembolization include liver failure, liver abscess, liver infarction, biloma, cholecystitis, and unintentional embolization to extrahepatic organs.16 Prophylactic intravenous antibiotics are routinely used. Coil embolization of the gastroduodenal artery may be required to prevent inadvertent chemoembolization of the pancreas and duodenum. A postembolization syndrome may occur that may include fever, nausea, and pain for several days following the procedure.
Cholangiocarcinoma
Cholangiocarcinoma is the only primary liver tumor that commonly causes vascular encasement and arterial occlusion.17 Encasement of the hepatic artery and splenic artery is seen frequently in cases of pancreatic carcinoma (Fig. 31-3).
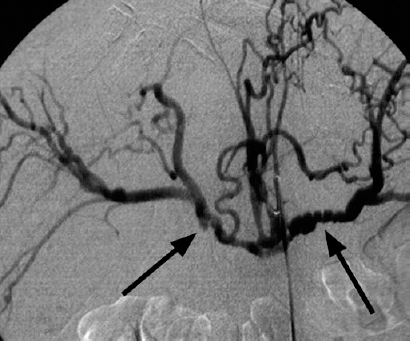
FIGURE 31-3. Hepatic artery and splenic artery encasement by pancreatic carcinoma. Irregular “sawtooth” narrowing of the hepatic and splenic arteries (arrows) is caused by tumor encroachment on the arterial walls.
Metastases
Hepatic metastases arise from hypervascular or hypovascular primary tumors and usually reflect the vascularity of the primary lesion. Unlike HCC, metastases typically are not associated with arteriovenous shunting or portal venous occlusion. The most frequent tumors that metastasize to the liver arise from the gastrointestinal tract (Fig. 31-4). Currently, hepatic resection is the only potentially curative therapy for patients with colorectal cancer metastases.18 The use of hepatic arterial chemotherapy infusion and chemoembolization for colorectal metastases is being investigated.19–21
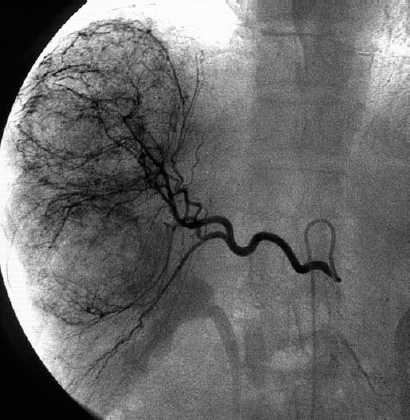
FIGURE 31-4. Metastasis to liver from colon carcinoma.
Resection of hepatic metastases has been associated with prolonged survival, particularly for metastases from colorectal primaries.22 Patients with more than four hepatic metastases, involvement of both hepatic lobes, or an estimated remaining liver that is less than 30% of the initial liver volume are not considered good candidates for resection.23
Computed tomography arterial portography and MRI are the imaging studies used to determine the extent of hepatic metastases. CT arterial portography is based on portal enhancement of the liver by infusion of contrast material through the superior mesenteric artery or splenic artery. Because all the injected contrast medium is delivered to the liver from the portal vein, the enhancement of the disease-free liver is high. Hepatic tumors generally do not have a portal venous blood supply, and they are detected as areas of low attenuation compared with the normal enhanced liver.
Neuroendocrine tumors
The two most common neuroendocrine tumors that metastasize to the liver are carcinoid and islet cell tumors. These are slow-growing neoplasms that frequently produce hormonal substances. The appendix is the most common site or origin of carcinoid tumors. Islet cell tumors typically arise from the pancreas. Patients with hepatic metastases from neuroendocrine tumors may remain relatively free of symptoms until the tumor replaces a significant volume of liver parenchyma. Symptoms are caused by production of hormones or mass effect. Surgical resection of neuroendocrine tumors is not a therapeutic option because these tumors are often metastatic at presentation. Hepatic artery embolization or chemoembolization is a recognized method of controlling hormonal symptoms and tumor bulk.24–26 Patients with carcinoid tumor refractory to other therapy can experience considerable relief of symptoms by such treatment. In addition, somatostatin is used during and after chemoembolization to prevent a carcinoid crises in hormonally active tumors. Somatostatin also can be used intravenously in the event of a carcinoid crisis.
Cavernous hemangioma
Cavernous hemangiomas are benign hepatic tumors that consist of thin-walled, endothelium-lined, septated vascular spaces. Cavernous hemangiomas frequently are identified incidentally during imaging. Hemangiomas are the most common benign tumors in the liver with an incidence of up to 7%. It is estimated that 70 to 95% of hemangiomas occur in women. Multiple hemangiomas have been identified in 10% of cases.27 Rarely, a large hemangioma (>4 cm) may cause symptoms from mass effect or bleeding.28
Hemangiomas may be seen on ultrasound as hyperechoic masses. On dynamic contrast-enhanced CT, hemangiomas initially appear hypodense and then show peripheral areas of focal enhancement, followed by diffuse hyperdensity at 2 minutes, and finally isodense on delayed scans. Red cell radionuclide scanning also can be used to make a reliable diagnosis. Hemangiomas produce intense signal on T2-weighted MR images.29 Rare cavernous hemangiomas may not exhibit conventional features and require needle biopsy for diagnosis.
Angiographic features of cavernous hemangiomas include a well-marginated tumor arising in the late arterial or capillary phase and persisting into the venous phase with early peripheral opacification. Cavernous hemangiomas have a normal-sized feeding artery with no neovascularity or arteriovenous shunting (Fig. 31-5).
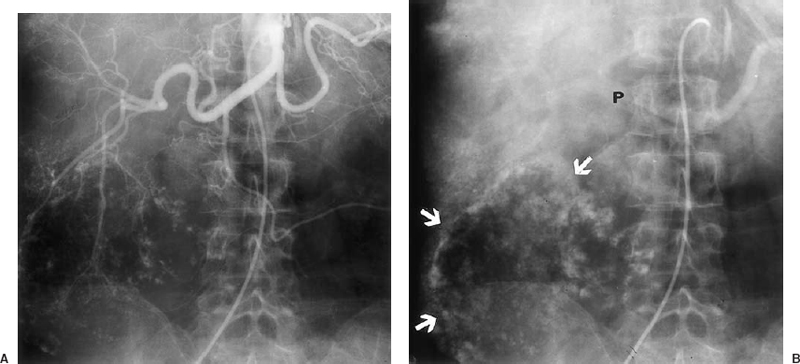
FIGURE 31-5. Giant cavernous hemangioma. A: Arterial Phase. B: Venous phase of a celiac trunk injection shows progressive opacification of the tumor periphery (arrows). P, portal vein. The feeding hepatic artery branches are of normal size with no hypervascularity or arteriovenous shunting.
Focal nodular hyperplasia
Focal nodular hyperplasia (FNH) is a benign tumor that contains liver elements, including hepatocytes, Kupffer cells, and bile ducts. FNH occurs in young women, is asymptomatic, and is not associated with oral contraceptive use; nor is it associated with malignant degeneration or spontaneous hemorrhage. An enhancing central scar is sometimes seen on CT or MR, but findings in FNH are typically nonspecific.30 The angiographic features include a well-marginated hypervascular tumor with feeding vessels entering from the periphery and a dense parenchymal stain (Fig. 31-6). There is no neovascularity or arteriovenous shunting present. There may be a central scar resulting in a “spoke-wheel” appearance.
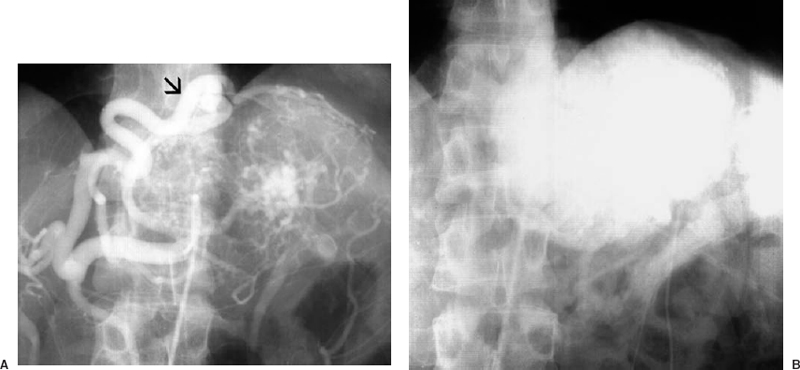
FIGURE 31-6. Focal nodular hyperplasia. (A). Large feeding artery (arrow) at the periphery of the lesion. (B). Dense parenchymal stain.
Hepatic adenoma
Hepatic adenoma is a neoplasm that is associated with hemorrhage caused by spontaneous necrosis. There exists a small potential for malignant degeneration. Most hepatic adenomas arise in young women, and there is a strong association with the use of oral contraceptives.31 Because of hemorrhage and necrosis, most adenomas are symptomatic when discovered. The CT and angiographic appearances of hepatic adenomas are nonspecific.
Portal hypertension
Cirrhosis is characterized by hepatic necrosis, regeneration, and fibrosis. In early cirrhosis, fibrosis develops around the sinusoidal spaces and obstructs central veins while preserving portal venules. Portal venous hypertension results from an increase in the vascular resistance within the liver. The portal venous system is decompressed through enlarged portosystemic collateral channels.
Several hemodynamic changes in the portal venous system occur as cirrhosis progresses. The most important is the enlargement of portosystemic collateral vessels. The direction of flow in the portal vein also changes as portal hypertension worsens. With mild cirrhosis, portal vein flow is hepatopetal. As resistance increases, bidirectional flow in the portal vein may develop, and the portal vein may not fill at all. With severe portal hypertension, the portal vein becomes an outflow conduit for the liver, and flow is hepatofugal. In patients with advanced cirrhosis, the hepatic arteries may have a corkscrew appearance because of increased arterial flow and liver shrinkage. As hepatic fibrosis worsens and portal flow decreases, cirrhotic patients become increasingly dependent on arterial perfusion of the liver.
The most common complication of portal hypertension is bleeding from gastroesophageal varices (Fig. 31-7). Normally, the portosystemic gradient is less than 5 mm Hg. Portal hypertension is defined as a portosystemic gradient of 6 mm Hg or greater. The risk of bleeding from gastroesophageal varices becomes significant when the gradient is 12 mm Hg or greater.32,33 Between 40 and 70% of patients die of the first episode of variceal bleeding. Other complications of cirrhosis and portal hypertension include ascites, hepatic encephalopathy, hepatorenal syndrome, bacterial peritonitis, splenomegaly, pancytopenia, hepatocellular carcinoma, and fulminant hepatic failure.
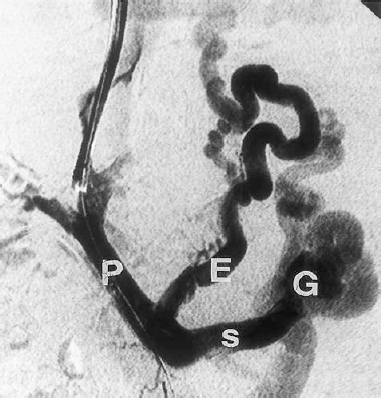
FIGURE 31-7. Gastroesophageal varices. Portal venogram during a TIPS procedure. P, portal vein; S, splenic vein; E, esophageal varices; G, gastric varices.
Management of acute variceal hemorrhage from portal hypertension traditionally included the use of pharmacologic agents, mechanical compression with tamponading balloons, and endoscopic techniques that include sclerotherapy and variceal banding. Intravenous infusions of medications—including vasopressin, nitroglycerin, propranolol, and octreotide—have been used for temporary control of variceal bleeding. Systemic infusion is as effective as intraarterial administration. Endoscopic banding and sclerotherapy are effective techniques for the initial management of variceal hemorrhage in most patients; however, sclerotherapy and banding do not correct portal hypertension.
Surgical treatments for portal hypertension include ligation and portosystemic shunt creation. Surgical shunt procedures provide long-term prevention of variceal hemorrhage by reducing portal venous pressure; however, surgical mortality of operative shunts has been reported to be as high as 20%.34 Liver transplantation is the definitive treatment for relieving portal hypertension from chronic liver disease.
Transjugular intrahepatic portosystemic shunts
The transjugular intrahepatic portosystemic shunt (TIPS) procedure was developed to relieve portal hypertension without the mortality and morbidity of an open surgical procedure. In this percutaneous procedure, an expandable metallic stent is placed in the liver to create a channel between the portal vein and hepatic vein (Fig. 31-8).
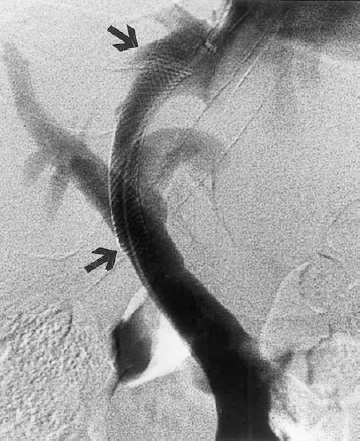
FIGURE 31-8. Transjugular intrahepatic portosystemic shunt. Wallstent extends from the portal vein to the hepatic vein (arrows).
The most common indications for the TIPS procedure include acute or recurrent variceal bleeding unresponsive to medical therapy, including endoscopic banding and sclerotherapy. Preliminary endoscopic examination is mandatory because bleeding actually may arise from nonvariceal causes such as peptic ulcer disease, alcoholic gastritis, esophageal ulcer, and Mallory–Weiss tear. TIPS has been performed to treat intractable ascites and to treat portal hypertension from Budd–Chiari syndrome.
Contraindications for the TIPS procedure include severe hepatic failure, severe right-sided heart failures, severe hepatic encephalopathy, primary or metastatic liver tumor, polycystic liver disease, portal vein thrombosis, and severe active infection.
TIPS procedure
Duplex sonography is performed before the procedure to establish portal and splenic vein patency and flow direction, to determine the status of the hepatic veins, to assess liver size, and to exclude the presence of a liver tumor or polycystic liver disease.
Access for the TIPS procedure usually is gained through the right internal jugular vein. A vascular sheath is passed into the right hepatic vein. The portal vein catheter–needle access system then is inserted through the sheath and passed from the hepatic vein through liver parenchyma into a branch of the intrahepatic portal vein. Several methods are used to select a site for puncture from the hepatic vein toward the portal vein. The most commonly used techniques are the use of bony landmarks and wedged injection of iodinated contrast material or carbon dioxide, which usually fills the central portal venous system.35–37
The needle access system then is exchanged over a guidewire for a diagnostic catheter. Portal venography and pressure measurements then are obtained. The parenchymal tract from the hepatic vein to the portal vein then is dilated with an 8-mm angioplasty balloon. A 10- or 12-mm diameter self-expandable Wallstent is deployed across the parenchymal tract. The stent then is dilated with a 10- or 12-mm balloon. Additional stents may be required to cover the entire tract from the hepatic vein to the portal vein.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree