Introduction
Shear wave elastography (SWE) assesses stiffness, not fibrosis. In addition to fibrosis, there are some factors or clinical conditions that may lead to an increase of liver stiffness (LS). They are known as confounding factors for fibrosis staging: liver inflammation, mostly gauged using transaminase values, which are indirect biomarkers; acute hepatitis; obstructive cholestasis; liver congestion; and infiltrative liver diseases.
The first report of an increase in LS in a patient with hepatic vascular congestion due to cardiac insufficiency dates back to 2008. The authors describe the case of a patient infected with hepatitis C virus after two heart transplants for severe ischemic cardiopathy followed by primary nonfunction of the graft. The patient had heart failure and showed a very stiff liver measuring 44.3 kPa at vibration controlled transient elastography (VCTE), with signs of cardiac hepatopathy but without liver cirrhosis at histology. One year after a third heart transplantation, a liver biopsy showed that there was a significant improvement of the cardiac hepatopathy, and the VCTE value was 3.8 kPa (i.e., within the normal range). The authors highlighted that vascular hepatic congestion can considerably increase LS up to values that are definitely diagnostic for liver cirrhosis. They also underscored that this increase is entirely reversible upon correction of cardiovascular dysfunction.
Since that report, several studies have investigated the role of the LS in patients with congestive heart disease and without a primary liver disease.
Heart diseases
Any disease affecting the right heart may lead to an increase of the pressure in the right atrium, the inferior vena cava (IVC), and the hepatic veins. Hepatic veins do not have valves, thus an increase of pressure in the IVC directly affects the sinusoidal bed, causing centrilobular congestion and sinusoidal dilation. The liver is covered by a minimally distensible but nonelastic capsule; therefore hepatic congestion may lead to an increase of stiffness.
Congestive heart disease
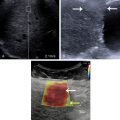
Heart failure is a major health problem with a considerable risk of morbidity and mortality. In patients with congestive heart disease, right-heart catheterization is the gold standard to measure central venous pressure (CVP). However, the procedure is invasive, not readily available, and not useful for following up patients. An indirect noninvasive parameter of the right atrial pressure (RAP), recommended by the guidelines of the American Society of Echocardiography, is the IVC diameter and its changes with breathing ( Fig. 9.1 ). The guidelines recommend IVC diameter be measured just proximal to the entrance of hepatic veins. IVC diameter ≤2.1 cm that collapses >50% with a breath suggests normal RAP (3 mmHg; range, 0–5 mmHg), whereas IVC diameter >2.1 cm that collapses <50% with a breath suggests high RAP (15 mmHg; range, 10–20 mmHg). If IVC diameter and collapse do not fit this paradigm, an intermediate value (8 mmHg; range, 5–10 mmHg) may be inferred or other indices of RAP should be integrated to downgrade to normal or upgrade to high RAP values. It is also underscored that in young athletes the IVC may be dilated in the presence of normal pressure and that the IVC is commonly dilated and may not collapse in patients on ventilators, so it should not be used in such cases for RAP estimate.
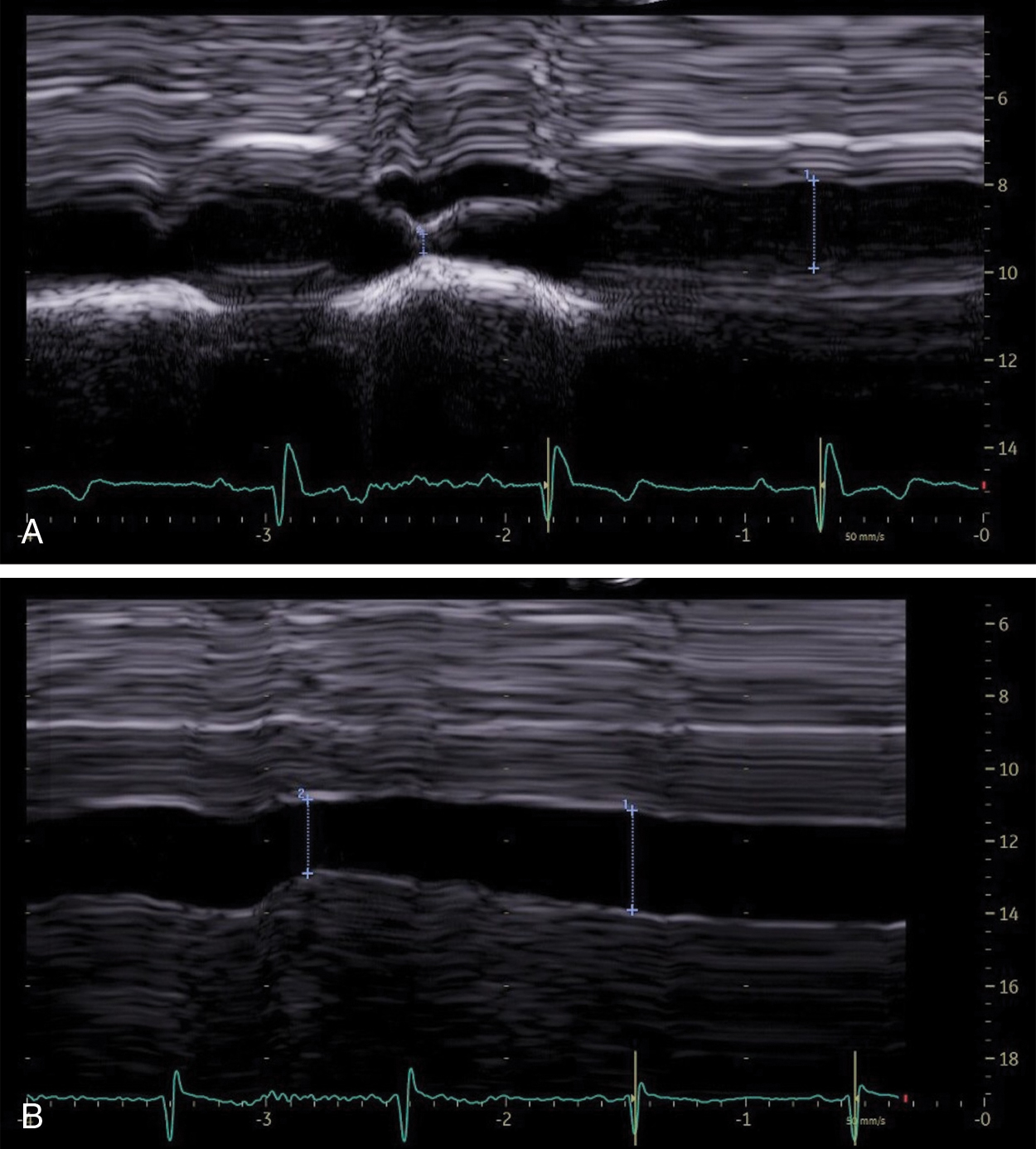
In patients with right-sided heart failure, the LS measurement could be a useful parameter for assessing RAPs and can be repeated over short periods of time.
Millonig and coworkers published the pivotal study in 2010 reporting that the LS is directly influenced by CVP. They showed that the clamping of the IVC in landrace pigs created a visible swelling of the liver and an increase in LS from 3.9 to 27.8 kPa. The reopening of the vein led to a rapid decrease in LS down to 5.1 kPa within 5 minutes ( P < 0.05). The reversible elevation of LS by increased venous pressure was highly reproducible in all five animals, suggesting that LS is directly controlled by the intravenous pressure in the absence of fibrosis or other causes of LS. For a direct correlation between hydrostatic pressure and LS, the authors also used the model of an isolated pig liver by clamping the portal vein, hepatic artery, and IVC distal and proximal to the liver. The IVC was then cannulated and the intravenous hydrostatic pressure was increased by infusion of isotonic saline solution. LS linearly increased with increasing intravenous pressures, and at a 36-cm water column, the maximum measurable LS with VCTE. (i.e., 75 kPa) was reached. The increase in LS was completely reversible and almost reached initial levels (5.5 kPa) within 5 minutes after resetting hydrostatic pressure back to a 0-cm water column.
Moreover, in a group of 10 patients with decompensated congestive heart disease that clinically recovered over a mean hospitalization interval of 7.2 days, they found a significant decrease of the LS from a median initial LS of 40.7 to 15.3 kPa. Thus treatment of cardiac insufficiency by diuretic therapy, concomitant weight loss, and clinical resolution of edema resulted in a decrease of initially elevated LS.
The decrease of the LS in patients with congestive heart disease who had a clinical improvement after treatment was confirmed in another small series of patients.
A study reported that LS could be an indirect marker of the RAP in patients with right-sided heart failure that was more accurate than IVC findings. In the study, both LS and IVC parameters were obtained within 3 hours before right-sided cardiac catheterization. There was a high correlation (r) between LS and RAP assessed with right-heart catheterization (r = 0.95), and the regression equation to predict RAP was −5.8 + (6.7 × natural logarithm of LS value in kilopascals). The authors found that an LS cutoff value of 10.6 kPa (with VCTE) identified RAP >10 mmHg with sensitivity and accuracy higher than the IVC parameters (sensitivity 0.85 vs. 0.56, accuracy 0.90 vs. 0.74, P < 0.05 for both).
The authors outline that IVC diameter and respiratory variation offer only semiquantitative assessment of RAP and may lead to erroneous inference, especially in patients with intermediate values, whereas LS gives a quantitative assessment of RAP and remains reliable even in patients on mechanical ventilation and with severe tricuspid regurgitation in which the use of echocardiography is usually limited.
However, it should be noted that 16 of the 105 (15.2%) patients who were screened were excluded. This is not a negligible percentage and may raise concern on the applicability of the technique in this setting. On the other hand, it should be highlighted that congestive heart disease may lead to organic liver disease, and this latter may be a confounder when the LS is used to noninvasively assess the CVP. For that reason it is of utmost importance to exclude cases with suspected organic liver disease in research studies.
Despite the improvements in the management of heart failure, there is still a high rate of hospital readmission due to heart failure. The same group of the previous study has assessed the prognostic value of the LS by VCTE in a series of hospitalized patients with heart failure. Of the 226 patients who were screened, 55 (24.3%) were excluded (37 for organic liver disease and 18 for invalid LS measurement). The LS was assessed before discharge in the remaining 171 patients and was stratified into three groups on the basis of the LS value: Group1: ≤4.7 kPa, corresponding to a RAP of 4.6 mmHg on the basis of the regression equation found in their previous study ; Group2: 4.7 to ≤6.9 kPa, estimated RAP 6.9 mmHg; Group3: >6.9 kPa, estimated RAP ≥7.0 mmHg. The authors found that the patients in Group3 were in the advanced New York Heart Association functional class and that they had a significantly higher risk of death or readmission to the hospital for heart failure than those in the other two groups. The LS value was able to predict cardiac events with a hazard ratio of 1.13 per 1-kPa increase in LS. LS showed a good predictive value for worse outcomes in patients regardless of severity of diastolic dysfunction. IVC diameter was also associated with the incidence of cardiac events; however, this parameter did not show significant predictive ability in the model that included both LS and IVC.
An LS value of 10.1 kPa had 0.73 sensitivity and 0.90 specificity for predicting worse short-term cardiac events. Notably, LS showed an incremental prognostic value when combined with previously established variables for predicting worse outcomes, including B-type natriuretic peptide. The results of this study indicate that the value of LS at discharge in patients with heart failure can be used as a reliable indicator of subclinical residual liver congestion, which reflects the severity of heart failure and adverse cardiac events, even in patients with optimized treatment and without visible edema or elevated liver function tests.
The prognostic value of the LS in patients with decompensated heart failure has been investigated in a few studies. Of note, the applicability of the technique in these studies is affected by the presence of other factors that could likely increase the LS, and invalid LS measurement or patients lost to follow-up should also be taken into account. In a study that excluded some 30% of patients for the previously mentioned reasons, 105 patients with acute decompensated heart failure were divided into two groups using an arbitrary LS value of 8.8 kPa by VCTE. In a median follow-up period of about 5 months, cardiac events (i.e., death or readmission to hospital) occurred in 54% of patients with LS ≥8.8 kPa and 25% of patients with LS <8.8 kPa ( P = 0.001). After adjusting for age, sex, and indices related to organ congestion, an LS ≥8.8 kPa was still significantly associated with cardiac events.
Another study assessed LS with VCTE both on admission to the hospital and the day of discharge in a series of 149 patients with acute decompensated heart failure. Overall there was a significant decrease of the LS during the hospitalization. An LS value higher than 13 kPa on admission and an LS value higher than 5 kPa at discharge was associated with an increased risk of 1-year all-cause death or readmission to the hospital. The authors highlight that LS increase is a nonspecific sign, therefore it would be difficult to use as the method for the differential diagnosis of liver dysfunction. However, in the clinical setting of an already established diagnosis of heart failure, after excluding other confounding factors, this limitation is not so relevant, and LS increase and its associations with negative prognosis in acute decompensated heart failure may be interpreted through two interrelated mechanisms of congestive hepatopathy: parenchymal congestion and congestion-induced fibrosis, both related to unfavorable outcomes.
Using a point shear wave elastography (pSWE) technique in patients with heart failure, it was reported that the changes in LS in patients with heart failure significantly correlated with changes in CVP in multivariate analysis and that an LS cutoff value of 7 kPa could predict a CVP >10 mmHg with 89.6% sensitivity and 87.5% specificity. , Another group reported that a high LS value on admission was an independent determinant of worse clinical outcomes in patients with acute decompensated heart failure.
All the published studies confirm that LS can be a marker of congestive heart failure; however, it is not yet clear what cutoff value should be used to define the risk of adverse cardiac events. In fact, this value ranges from >5 to 8.8 kPa. Of note, the “rule of 5” proposed by the Baveno VI consensus on portal hypertension has proposed that LS value with VCTE up to 5 kPa may exclude liver fibrosis.
It should also be emphasized that there is an interaction between the liver and the heart: heart failure and liver disease often coexist, because of systemic disorders and factors/diseases that affect both organs (alcohol abuse, drugs, inflammation, autoimmunity, infections). Moreover, there is a complex interaction between the heart and the liver: heart failure may lead to irreversible liver disease; conversely, liver disease may cause cardiac dysfunction and failure in the absence of other cardiovascular abnormalities. Therefore, in some cases, the increase in LS may be due to both liver congestion due to heart disease and liver disease, even when other causes of primary liver disease are excluded. Extensive fibrosis can be seen in chronic or severe cases of congestive hepatopathy. On this regard, it is worth mentioning that liver biopsy data were not available in the majority of the studies reported previously.
A study in a small series of patients with end-stage chronic heart failure who underwent left ventricular assist device implantation reported that the LS values were affected both by the central venous congestion and by the histologic changes of the liver. On the other hand, in patients with severe heart failure who required a left ventricular assist device, it has been observed that the incidence of major adverse events was lower when the LS was ≤12.5 kPa.
Congenital heart diseases and valvular heart diseases
The usefulness of LS as a noninvasive tool for the evaluation of CVP in patients with congenital heart disease and valvular disease has been assessed in several studies.
One study that included both children and adults with congenital heart disease undergoing cardiac catheterization reported that LS significantly correlated with CVP (r = 0.75). When the two subgroups were analyzed separately, the correlation was significantly higher in the adults (r = 0.68 in children vs. r = 0.84 in adults, P < 0.0001). Overall, the area under the receiver operating characteristic (AUROC) curve of the LS for identifying a CVP >10 mmHg was 0.97, and the optimal cutoff value of LS for detection of CVP >10 mmHg was 8.8 kPa with 92% sensitivity and 96% specificity. Considering the children separately (n = 60) from adults (n = 36), the AUROC curve of LS for identification of CVP >10 mmHg was 0.99 with an optimal LS cutoff value of 6.8 kPa (100% sensitivity, 90.5% specificity) in children and 0.95 with an 8.8 kPa cutoff value (100% sensitivity, 89% specificity) in adults.
The LS with a two-dimensional shear wave elastography (2D-SWE) technique was measured in 79 patients aged <20 years old with congenital heart disease and without liver disease who underwent cardiac catheterization. Of them, 34 (43.0%) had Fontan physiology. As observed in adult patients with heart failure, CVP was the only factor that independently and significantly correlated with LS (r = 0.78). This correlation was also present in the subgroups of patients with biventricular disease and those who underwent the Fontan procedure.
In a series of 131 patients with various degrees of tricuspid valve regurgitation secondary to left-sided heart valve disease, it was found that the individuals with severe regurgitation had higher LS than those with moderate regurgitation. Moreover, the LS values were associated with the parameters that noninvasively assess the severity of tricuspid regurgitation, such as the area of the regurgitant orifice, the RAP, and the IVC diameter.
The change over time of the LS value was assessed in a series of 32 consecutive patients undergoing surgery for valve replacement or repair. All patients had tricuspid valve regurgitation secondary to left-sided heart valve disease. It was reported that LS decreased significantly at 3 months after corrective surgery, from 8.4 to 6.0 kPa ( P = 0.03).
Therefore LS could be proposed as a tool to evaluate, noninvasively, the effects of medical therapy, transcatheter interventions, or mechanical devices on CVP.
Fontan circulation
The Fontan operation was proposed for the surgical repair of tricuspid atresia, and it still is the palliative standard procedure for patients with univentricular physiology. The Fontan procedure leads to physiological pulmonary blood flow restoration obtained by diverting the inferior and superior vena cava blood to the pulmonary arteries through cava-pulmonary anastomoses. Doing so, the right atrium is “ventriclized” and oxygenated blood only returns to the left heart. In a Fontan circulation there is no pump to propel blood into the pulmonary arteries, because the systemic veins are directly connected to the pulmonary arteries, and there is a critical bottleneck with obligatory upstream congestion and downstream decreased flow that account for most of the clinical and physiological disorders in a Fontan circuit. The elevated pressure is transmitted to the liver directly through the IVC and the hepatic veins.
The advances in the surgical techniques and in the management of patients with single ventricle physiology, congenital heart disease, and Fontan circulation have led to a longer survival of Fontan-palliated patients who now reach adulthood in the majority of cases.
However, the altered hemodynamics lead to disfunction of several organs, especially the liver. In fact, cardiac cirrhosis is quite common in Fontan-palliated patients during their adulthood. The pathologic changes in the liver are sinusoidal dilatation and fibrosis, likely due to high venous pressures, hypoxia, and diminished cardiac output.
Using a 2D-SWE technique in patients with Fontan circulation, a study demonstrated that the LS values correlated with the stage of histopathologic fibrosis and that the LS values were significantly higher (15.6 kPa vs. 5.5 kPa, P < 0.0001) than in healthy controls. Forty-one patients with Fontan physiology and 65 controls were enrolled, and a small number of Fontan patients underwent transjugular liver biopsy. LS on average was 13.4 kPa in patients with METAVIR fibrosis stage F <2 (n = 4) and 19.8 kPa in patients with F ≥2 (n = 6). These LS values are significantly higher than that observed in other causes of chronic liver disease likely due to the combination of fibrosis and congestion. It was also found that portal vein blood flow assessed with Doppler flowmetry was decreased, whereas the celiac and mesenteric arterial resistive indices were higher and that the LS correlated both with ventricular end-diastolic pressure and pulmonary artery wedge pressure as well as the degree of liver fibrosis. All these findings clearly indicate there is interplay between the hepatic afterload and the histological changes.
Hepatic complications are correlated with the duration of the Fontan circulation. Using VCTE, a study in 39 patients with Fontan circulation reported a significant correlation between the stage of fibrosis and the interval of time since the Fontan surgery, with a sharp increase in the number of patients with significant liver fibrosis at 5 years after the operation. Moreover, there was a relationship between the LS and the inspiration/expiration diameter ratio of the IVC (i.e., a sign of liver congestion).
A study performed with a pSWE technique in 64 Fontan patients demonstrated that patients with a fenestration had significant lower mean LS than patients without (1.75 vs. 2.03 m/s, P = 0.003).
A study in a small series of Fontan patients (n = 10) with liver biopsy results available reported that the VCTE cutoffs obtained in patients with mixed causes of chronic liver disease would have overestimated fibrosis by at least one stage in 70% of subjects and by at least two stages in 50% of subjects. There was no agreement with liver biopsy results when using the 2D-SWE cutoffs obtained in patients with chronic hepatitis C in a small cohort that included 14 Fontan patients who underwent liver biopsy.
The Fontan procedure leads to a significant increase in LS caused by hepatic congestion. , This increase persists chronically, therefore the evaluation of liver fibrosis in this setting is challenging because it is not possible to separate the two factors (i.e., congestion and fibrosis) and it could lead to an overestimation of fibrosis.
Using pSWE in 18 children undergoing the Fontan operation (stage 3), an immediate marked increase in LS from 1.18 to 2.28 m/s, on average, simply due to increased hepatic systemic venous afterload and increased CVP was observed; this increase in LS persisted to the time of hospital discharge and beyond. In a small series (9 children; age range, 3.5–5.6 years), it was observed that LS increased from 6.2 ± 1.5 kPa in the preoperative period to 11.2 ± 4 kPa at a mean follow-up of 4 months.
For the follow-up and longitudinal monitoring of Fontan patients, the assessment of the delta changes in LS over time as suggested by the update to the Society of Radiologists in Ultrasound consensus statement can be applied and provides valuable information. This approach helps overcome the lack of reliable cutoff values and our current inability to distinguish if the increased stiffness is caused by congestion or fibrosis. An increase of LS over time is indicative of worsening fibrosis and/or congestion and may indicate additional clinical investigation is needed.
Liver diseases beyond the staging of fibrosis
LS assessment is a reliable and noninvasive method for the staging of liver fibrosis in several clinical scenarios, and it is an accepted biomarker of portal hypertension. , , The potential role of LS in evaluating liver disease beyond the stage of liver fibrosis has been investigated in hepatic sinusoidal obstruction syndrome (SOS), Budd-Chiari syndrome, and biliary atresia (BA).
Hepatic sinusoidal obstruction syndrome
Hepatic SOS, previously named hepatic venoocclusive disease, is caused by toxic damage to the hepatic sinusoidal endothelial cells that leads to loss of sinusoidal wall integrity, endothelium detachment, and embolization toward the centrolobular zone of the hepatic acinus. , These events hamper the liver blood outflow, with sinusoidal obstruction resulting in congestion and ultimately development of postsinusoidal portal hypertension.
SOS may be a result of cytoreductive therapy prior to hematopoietic stem cell transplantation following oxaliplatin-containing adjuvant or neoadjuvant chemotherapy for colorectal liver metastases, after the ingestion of alkaloid toxins, and in other particular settings such as the autosomal recessive condition of venoocclusive disease with immunodeficiency or after high-dose radiation therapy.
SOS is a life-threatening disease in its severe forms. The diagnosis is based on clinical criteria including weight gain, hepatomegaly, right-upper quadrant pain, ascites, and jaundice. The use of defibrotide for the treatment of severe forms has reduced the high mortality rate. The mortality is lower when the treatment is started earlier, therefore an earlier diagnosis is of utmost importance. On this regard, studies suggest that elastography could play an important role.
In 2011 Fontanilla and coworkers reported an increase of LS assessed by a pSWE technique (2.75 m/s and 2.58 m/s) in two adult patients diagnosed with SOS. They observed that LS decreased to normal values after successful treatment.
The potential role of LS assessment in this setting has been investigated in rat models of acute and severe SOS or chronic, mild, and reversible SOS. In both SOS models, LS values were significantly higher than in the matched control rats. In the chronic and reversible SOS models there was a significant decrease of LS after a treatment-free period of 2 weeks.
The role of LS in predicting SOS syndrome has been investigated in a series of 25 pediatric patients who received hematopoietic stem cell transplant and in those whom LS by pSWE technique was assessed at three scheduled time points. Five of them developed SOS. In respect to the patients who did not develop SOS, they had a significant increase of LS at day +5 and at day +14 after transplant. LS increase occurred on average 9 and 11 days before clinical and conventional ultrasound (US) diagnosis of SOS. Therefore an LS increase seems to be an early marker of SOS development, allowing an early diagnosis and the possibility to timely start an effective treatment.
Similar findings were observed in a series of 78 adult patients who underwent hematopoietic stem cell transplantation. The median baseline LS value assessed by VCTE was 4.2 kPa. Four patients (5.1%) presented with SOS, and in all of them LS showed a significant increase in respect to baseline values 2–12 days before the clinical manifestation of SOS. The three patients who were successfully treated with defibrotide showed a decrease of LS, which reached pretransplantation value within 2–4 weeks after the diagnosis of SOS, whereas in the patient with severe SOS who died 20 days after the diagnosis of SOS, there was not any decrease of LS.
An algorithm has been proposed for the diagnostic work-up of patients undergoing hematopoietic stem cell transplantation. A baseline assessment with US and SWE is recommended within 1 month before the procedure. As per the guidelines, after the transplantation the patient must be screened daily to rule out the onset of SOS. US and SWE should be performed weekly to detect any morphological, Doppler, and LS changes. In the absence of clinical signs but with a sudden increase in stiffness compared with the baseline value, a close clinical follow-up is required and could be combined with US or radiological (magnetic resonance imaging [MRI]/computed tomography [CT]) studies. This follow-up is needed because there is a high likelihood that SOS will develop in the following days. If there is an agreement between the clinical evaluation and the radiological findings, the diagnosis of SOS can be confirmed; otherwise, an invasive approach is necessary to confirm or rule out SOS.
Budd-Chiari syndrome
Budd-Chiari syndrome is due to hepatic venous outflow obstruction in the absence of cardiac or pericardial disease that leads to hepatic congestion and portal hypertension. The outflow obstruction can be partial or complete and may occur in small or large hepatic veins, the suprahepatic segment of the IVC, or the right atrium. The clinical manifestations are highly variable, ranging from fulminant or acute liver failure to subacute, chronic, or asymptomatic forms. Important clinical features include abdominal pain, ascites, hepatosplenomegaly, and prominent venous collaterals secondary to the obstruction of the IVC. Budd-Chiari syndrome can be primary or secondary, depending on the cause of the hepatic venous outflow obstruction. It is classified as secondary when the hepatic flow is obstructed by compression or invasion of a lesion outside the hepatic venous outflow. Primary cases are, in the majority of cases in the Western world, caused by an underlying hypercoagulable or prothrombotic state resulting from several congenital conditions (such as protein C deficiency, protein S deficiency, antithrombin deficiency, factor V Leiden mutation) or myeloproliferative disorders, paroxysmal nocturnal hemoglobinuria, hyperhomocysteinemia, Behçet’s disease, oral contraceptive intake, antiphospholipid antibody, malignancy, and few other conditions. Anatomical anomalies of the IVC, such as membranous obstruction, are less frequent and more common in Asian populations.
Noninvasive imaging such Doppler ultrasonography, CT, or MRI play a crucial role in the diagnosis of Budd-Chiari syndrome, demonstrating the hepatic venous outflow.
Rather than a diagnostic tool, SWE can potentially be used in the follow-up of patients. Few studies have been published so far.
The role of LS in monitoring short- and long-term outcome after angioplasty was assessed in a series of 25 patients with Budd-Chiari syndrome. There was a significant decrease of LS values within 24 hours after intervention from 62.8 to 26.3 kPa. In the patients for whom liver histology results were available, the changes in LS did not show any significant difference between cases with mild fibrosis and significant fibrosis. There was also a significant difference between LS values obtained at 24 hours and those at 3 months after treatment (26.3 kPa vs. 20.9 kPa; P = 0.003). It is suggested that LS measurement can be used as a surveillance tool to longitudinally assess the outcome of the vascular intervention, irrespective of preintervention fibrosis stage.
Likewise, a significant decrease of LS 2 days after the treatment from 35.2 to 20.1 kPa was observed in a series of 32 patients with Budd-Chiari syndrome successfully treated with angioplasty. The patients were followed up with for 6 months. With respect to baseline values, there was a significant decrease at 3 months; thereafter, LS values remained stable even though the liver was still in the cirrhotic stage.
In a case report, LS with the pulsed Doppler waveform of the hepatic veins was used to follow-up at 3-month intervals with a patient who presented with restenosis of the IVC after balloon dilation. A sharp increase of LS (from 14.3 to 20.5 kPa) and a monophasic pattern of the hepatic vein flow at pulsed Doppler were markers of restenosis confirmed by x-ray venography.
Biliary atresia
BA is a progressive fibroinflammatory process that causes obliteration of the extra- and intrahepatic biliary tree and that clinically presents during the first few weeks of life with persistent jaundice and elevated conjugated bilirubin. It is the most common cause of chronic cholestasis in infants, and it leads to secondary biliary cirrhosis in the first months of life and to death in early childhood if untreated. Kasai hepatic portoenterostomy (HPE) is the standard treatment for BA, and early HPE (before 46 days of life) results in a higher rate of survival with native liver and better long-term clinical outcomes, and thus a high index of suspicion is needed for investigation of infants with persistent jaundice. ,
The diagnosis of BA is often based on some combination of B-mode US imaging, hepatobiliary scintigraphy, liver biopsy, and intraoperative cholangiography. Early diagnosis is of utmost importance to reestablish bile flow from the liver into the small bowel and provide the best patient outcomes. Conventional B-mode US is recommended for BA diagnosis. It has a good diagnostic performance; however, the B-mode findings do not rule out nonsyndromic BA.
In BA the liver may become very stiff, and there is evidence in the literature that SWE can be used to help differentiate BA from other nonsurgical causes of neonatal jaundice.
In a study on 48 cholestatic neonates, 15 of whom had BA, an LS cutoff value of >7.7 kPa by VCTE had 80% sensitivity and 97% specificity for diagnosing BA.
A study in 41 patients, 13 of whom were diagnosed with BA, reported that LS by pSWE was significantly higher in patients with BA (1.95 vs. 1.21 m/s), and a cutoff value >1.53 m/s had 76.9% sensitivity and 78.6% specificity. In the same study, a cutoff value of LS by 2D-SWE >1.84 m/s had 92.3% sensitivity and 78.6% specificity.
The utility of LS measurement by 2D-SWE and several commonly used biomarkers in differentiating BA from other causes of cholestasis (non-BA) patients within 45 days and in predicting the postoperative prognosis has been investigated in a series of 156 patients, consisting of BA (n = 83) and non-BA (n = 73) cases. 2D-SWE and serum gamma-glutamyl transferase showed better discriminative utility. The optimal cutoff values for 2D-SWE and gamma-glutamyl transferase were >7.10 kPa and >195.4 U/L, with the AUROC curves of 0.82 and 0.87, respectively. Subgroup analysis showed an increased discriminative performance of 2D-SWE with age.
The survival rate with a native liver after a successful HPE is >50% at 10 years and 30%–40% at 20 years, whereas orthotopic liver transplantation has a 10-year survival rate of 86%. Literature data suggest that liver and spleen SWE could be a valuable tool to monitor liver disease and portal hypertension after the Kasai operation. In a study on 31 patients with BA who had undergone a Kasai portoenterostomy, the optimal LS cutoff by VCTE for predicting esophageal varices was >10.6 kPa (87% sensitivity, 87.5% specificity, and 0.92 AUROC curve).
In a series of 69 patients who underwent HPE and performed VCTE before and 3 months after HPE, LS value was the most powerful independent factor of the development of liver-related events. The cutoff value of 19.9 kPa had 85.3% sensitivity, 95.2% specificity, and 0.94 AUROC curve.