Chapter Outline
Indications for Liver Transplantation
Patient Evaluation for Liver Transplantation
Listing and Timing of Liver Transplantation
Model for End-Stage Liver Disease (MELD) Score
Hepatocellular Carcinoma and Liver Transplantation
Exclusion of Hepatocellular Carcinoma
OPTN Classification System for Nodules Seen on Imaging of Cirrhotic Livers
Locoregional Bridging Therapy for Hepatocellular Carcinoma
Living Donor Liver Transplantation
Orthotopic liver transplantation (OLT) is the only definitive treatment of end-stage liver disease. About 6000 liver transplants are performed annually in the United States. Lack of donors remains a huge challenge for liver transplantation programs. Of the 15,641 patients on the waiting list for liver transplantation in 2009, 2396 patients died waiting for a transplant. The first human liver transplant was performed in 1963 by Thomas Starzl. The surgical technique and management of patients with liver transplant significantly improved during the next few decades. Increasing experience has resulted in current 1-, 3-, and 5-year survival rates of 87%, 78%, and 73%, respectively. Early graft failure within 6 weeks of transplantation in deceased donors has decreased from 6.9% in 1998 to 3.0% in 2009.
In 1987, Congress passed the National Organ Transplant Act, which established the Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients. OPTN links all professionals involved in the organ donation and transplantation system. The United Network for Organ Sharing (UNOS) is the OPTN contractor that coordinates the nation’s transplant system. UNOS maintains the national organ transplant waiting list, increases awareness of organ donation, develops policies and standards for transplantation, and monitors members for compliance.
Indications for Liver Transplantation
The indications for OLT are presented in Table 92-1 .
| Primary Cause of Disease | Percentage |
|---|---|
| Hepatitis C | 25.6 |
| Malignant disease | 18.7 |
| Alcoholic liver disease | 17.4 |
| Cholestatic disease | 7.9 |
| Acute hepatic necrosis | 4.3 |
| Metabolic liver disease | 2.5 |
| Others (nonalcoholic steatohepatitis most common) | 23.6 |
Hepatitis C
Chronic liver disease caused by hepatitis C virus infection is the leading cause of liver transplantation. Unfortunately, reinfection of the allograft by the hepatitis C virus is universal. Reinfection results in faster progression to fibrosis and cirrhosis compared with the nontransplant population. Cirrhosis develops in 25% of recipients within 5 to 10 years of transplantation. Graft failure rate at 10 years is higher in patients with hepatitis C (45%) compared with the rate for all deceased donors (51.3%).
Alcoholic Liver Disease
Patients who undergo liver transplantation for alcoholic liver disease have outcome similar to that of patients receiving transplants for nonalcoholic disease. However, studies have shown that 10% to 15% of patients receiving transplants for alcoholic liver disease resume heavy drinking, which can harm the transplant. UNOS suggests at least 6 months of abstinence before transplantation (6-month rule). This effectively eliminates patients with acute alcoholic hepatitis from receiving transplants.
Malignant Disease
Hepatocellular carcinoma (HCC) occurring in the setting of cirrhosis is a common indication for transplantation. Cholangiocarcinoma, hemangioendothelioma, and hepatoblastoma are much rarer indications for transplantation.
Nonalcoholic Steatohepatitis
Nonalcoholic fatty liver disease represents a spectrum of liver disease due to triglyceride accumulation in the hepatocytes ranging from benign steatosis to nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis. As a consequence of the growing epidemic of obesity, nonalcoholic fatty liver disease and NASH have a prevalence of 30% and 12%, respectively, in the U.S. population. NASH represents a rapidly growing indication for liver transplantation in the last decade. It is currently the third most common cause of transplantation. In 2009, NASH was listed as the primary indication for 8.5% of liver transplants compared with 1% in 2001. These numbers are likely to underestimate the true incidence of NASH as underlying steatosis often dissipates after the development of cirrhosis. A substantial portion of patients with cryptogenic cirrhosis listed as the indication for transplantation probably had NASH.
Cholestatic Liver Disease
Primary biliary cirrhosis and primary sclerosing cholangitis are the most frequent cholestatic conditions in adults, and liver transplantation is an excellent option for patients who progress to end-stage disease. Transplantation for cholestatic disease has better survival rates than many other indications. Biliary atresia, cystic fibrosis, and Alagille’s syndrome are cholestatic conditions in children that can be treated with transplantation.
Acute Hepatic Necrosis
Acute hepatic necrosis or acute liver failure is characterized by sudden massive necrosis of hepatocytes and has very high mortality. Liver transplantation provides the most definitive treatment of acute liver failure. Viral hepatitis and drug toxicity (including acetaminophen) are common causes.
Metabolic Disease
Wilson’s disease, hereditary hemochromatosis, and α 1 -antitrypsin deficiency are metabolic diseases that can cause irreversible hepatic damage.
Patient Evaluation for Liver Transplantation
Listing and Timing of Liver Transplantation
The demand-supply situation for liver transplantation is such that a large number of patients are waitlisted for a small number of available organs. Before 2002, patients received transplants on a first-come, first-served basis. However, the sickest patients in the most urgent need of transplantation were not always the first on the list, and death on the wait list increased. The optimal timing of liver transplantation should be such that a patient must be sick enough to require a transplant but not be so sick as to be unable to withstand the transplantation. Comorbid conditions (like HCC) should not be allowed to advance so much that survival of the graft or of the patient is affected. The MELD score and Milan criteria are used to evaluate the need of transplantation.
Model for End-Stage Liver Disease (MELD) Score
The MELD score is a predictor of short-term mortality in patients with chronic liver disease. It is calculated from serum creatinine concentration, international normalized ratio, and bilirubin concentration. Since 2002, UNOS has used the MELD score to assess the acuity of need for liver transplantation. Patients with high MELD scores have more urgent need of transplantation and are placed higher in the list irrespective of their time of listing.
Hepatocellular Carcinoma and Liver Transplantation
HCC commonly arises in cirrhotic livers, particularly in patients with hepatitis B and C. Liver transplantation can potentially treat the underlying liver disease as well as the HCC. The selection of patients with HCC for liver transplantation is complicated. Patients with advanced HCC who undergo transplantation have shown disease recurrence and poor survival. In view of donor liver shortage, transplantation for patients with advanced HCC is not advisable as scarce organs could be directed to patients more likely to benefit from the transplant.
Patients with HCC may not have such poor synthetic function (or high MELD score) to qualify for a liver transplant. As these patients wait for their liver function to deteriorate enough to qualify for transplantation, the underlying HCC may advance such that the patient is no longer a good candidate for transplantation because of the risk of HCC recurrence.
To deal with this problem, UNOS/OPTN does not allow transplantation in patients with advanced HCC by the Milan criteria and gives exception points (based on certain criteria) to patients with limited HCC for additional priority for liver transplantation.
Milan Criteria
The Milan criteria ( Table 92-2 ) are used to identify patients with HCC in whom the tumor burden is small enough to allow good outcome after liver transplantation. The Milan criteria state that transplantation should be performed in those with a single tumor of 5 cm or less or three tumors that are each 3 cm or less, no macrovascular invasion, and no metastasis. Patients who do not meet the Milan criteria are not considered eligible candidates for liver transplantation. Some authors have suggested that the Milan criteria are too restrictive and that acceptable outcomes can be obtained with more liberal tumor criteria (e.g., University of California, San Francisco, criteria).
| Class | Size | |
|---|---|---|
| 5A | ≥1 cm but <2 cm | Increased arterial enhancement and washout and pseudocapsule |
| 5A-g | Same as 5A | Increased arterial enhancement and growth by 50% in 6 months |
| 5B | ≥2 cm but ≤5 cm | Increased arterial enhancement and either washout or pseudocapsule or growth by 50% in 6 months |
| 5T | Prior locoregional therapy for HCC | Describes any residual lesion or perfusion defect at prior UNOS class 5 lesion |
| 5X | >5 cm | Meets criteria for HCC but outside stage T2 and not eligible for exception points |
MELD Exception Points for Hepatocellular Carcinoma
For patients within the Milan criteria, UNOS/OPTN grants exception points to stage T2 HCC (one tumor ≥2 cm and ≤5 cm or two or three tumors ≥1 cm and ≤3 cm). This allows these patients to undergo transplantation earlier in the course of their disease in an attempt to achieve better long-term survival after transplantation. The goal is to prevent the patient from reaching an HCC tumor burden that exceeds the Milan criteria, which would necessitate the patient’s being removed from the transplantation waiting list.
Preoperative Imaging
The main objective of preoperative imaging is to provide the surgeon with the necessary information needed to plan and perform liver transplantation and to exclude patients for whom surgery either is not feasible or would be of no benefit. Preoperative radiologic evaluation is used to detect HCC or other malignant disease, to determine the size and patency of the portal vein and superior mesenteric vein, to assess hepatic arterial inflow, and to evaluate surgical portosystemic shunts and location of varices.
Exclusion of Hepatocellular Carcinoma
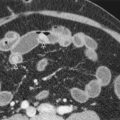
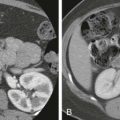
Patients awaiting liver transplantation undergo contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) to screen for HCC. Ultrasound is used for routine screening of cirrhotics for HCC, but patients being considered for a transplant usually undergo CT or MRI. A multiphasic technique with late arterial, portal venous, and delayed phases is used. HCCs derive their blood supply from the hepatic arteries and show hyperenhancement in the arterial phase. They “washout” or become hypoattenuating to the hepatic parenchyma in the portal venous and delayed phases. A peripheral rim of enhancement or “pseudocapsule” may be present on the portal venous or delayed phase ( Fig. 92-1 ). Any hepatic lesion identified is classified by the OPTN classification system. The number and size of each HCC and the presence of any vascular invasion or metastatic disease are noted ( Fig. 92-2 ).


OPTN Classification System for Nodules Seen on Imaging of Cirrhotic Livers
OPTN/UNOS has strict radiologic criteria for classification of nodules in cirrhotic livers as HCC. Only class 5 nodules are considered to meet radiologic criteria for HCC and may qualify for automatic exception, depending on stage (see Table 92-2 ).
Portal Vein Evaluation
The portal venous system can be assessed by Doppler ultrasound, CT, or MRI. Liver transplantation in patients with extrahepatic portal venous thrombosis or a small-caliber vein is difficult or sometimes impossible. The normal diameter of the extrahepatic portal vein is 8 to 12 mm. The extrahepatic portal vein usually needs to be at least 4 to 5 mm in diameter for a successful portal vein anastomosis. On Doppler examination, portal vein thrombosis is usually depicted as echogenic material within the portal vein. The extent of thrombus should be determined, and the entire extrahepatic portal vein and superior mesenteric vein should be evaluated. Although extensive portal vein thrombus was considered a contraindication to OLT in the past, many patients can successfully undergo liver transplantation with modified surgical techniques. In these patients, evaluation of the superior mesenteric vein is critical. If the superior mesenteric vein is patent, placement of an interposition graft or venous jump graft from the superior mesenteric vein to the portal vein is a feasible option. The hepatic veins and inferior vena cava (IVC) also should be evaluated for patency.
Hepatic Artery Evaluation
Preoperative assessment of recipient hepatic arterial supply is important to ensure adequate hepatic arterial inflow to the transplanted liver. The arterial patency can be assessed on preoperative ultrasound, CT, or MRI. Inadequate hepatic arterial inflow usually results from compression of the celiac trunk by the arcuate ligament or from atherosclerosis. In patients with arcuate ligament compression, simple division of the ligament improves blood flow. Compromised arterial inflow resulting from atherosclerosis usually requires creation of either a supraceliac or an infrarenal aorto–hepatic artery bypass graft.
Portosystemic Shunt and Variceal Evaluation
CT and MRI can precisely locate surgical and spontaneous portosystemic shunts and varices that may be encountered. In patients with cirrhosis, surgical portosystemic shunts are often created to decompress portal hypertension and varices to diminish the chance of gastrointestinal hemorrhage. When transplantation is performed, these surgical portosystemic shunts are often ligated so that portal flow to the allograft is not compromised. Likewise, the presence and location of varices are helpful to the surgeon. Varices that are encountered may be ligated. Alternatively, the surgeon may choose to avoid rather than to ligate certain varices, thereby reducing the potential for hemorrhagic complications.
Locoregional Bridging Therapy for Hepatocellular Carcinoma
Locoregional therapies are used to treat HCC in patients on the transplant waitlist. These are used as bridge strategies with the primary goal of reducing tumor progression to allow patients to be longer on the waitlist. Although bridge strategies are widely used, it is not clear whether they are effective in maintaining patients on the list or improving disease-free survival.
Image-guided transcatheter delivery of therapeutic agents through vessels feeding the HCC is a commonly used bridge therapy that allows more focused delivery of therapeutic material within the tumor. Conventional transcatheter arterial chemoembolization consists of intra-arterial infusion of a drug, such as doxorubicin or cisplatin with or without a viscous emulsion, followed by embolization of the blood vessel with gelatin sponge particles or other embolic agents. Recent advances include use of drug-eluting beads for more controlled and sustained drug release. Radioembolization by transcatheter infusion of microspheres coated with yttrium 90, a beta particle–emitting isotope, into feeding vessels allows delivery of high-energy, low-penetration radiation to the tumor.
Local ablation is a commonly used bridge therapy for small HCCs (single or few) that are accessible either percutaneously or laparoscopically. Multiple ablative techniques, including radiofrequency ablation, microwave ablation, cryoablation, and ethanol injection, are available. In patients with good hepatic function, surgical resection of a solitary HCC is also used as bridge therapy.
Surgical Technique
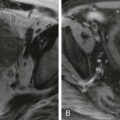
The main steps of an OLT procedure consist of donor hepatectomy, recipient hepatectomy, hemostasis, and vascular anastomoses followed by biliary anastomosis. The caval anastomosis is the first vascular anastomosis performed. The standard and piggyback techniques are different methods to perform the caval anastomosis ( Figs. 92-3 and 92-4 ). In the standard technique, the recipient’s retrohepatic IVC is removed with the recipient’s liver. End-to-end anastomosis is then performed twice between the donor and recipient IVC above and below the liver. In the newer piggyback technique, the recipient’s IVC is not removed with the recipient’s liver. The donor’s suprahepatic IVC is anastomosed to the common orifice of the recipient’s hepatic veins. The main advantage of the piggyback technique is that it preserves caval flow and reduces hemodynamic instability during the procedure.


The portal venous and hepatic arterial anastomoses are done after the caval anastomosis. The portal venous anastomosis is an end-to-end anastomosis. The arterial anastomosis is often performed with a Carrel patch. In this, the donor hepatic artery is harvested at the level of the celiac axis with a patch of the aorta. The aortic patch is then anastomosed to the recipient hepatic artery near the gastroduodenal artery takeoff.
The biliary anastomosis is usually an end-to-end choledochocholedochostomy. A biliary-enteric anastomosis (choledochojejunostomy) is performed in recipients with bile duct disease. Traditionally, biliary anastomosis has been performed with T-tube drainage, but some institutions have now moved away from routine use of T-tube drainage for deceased donors. Presence of a T tube allows monitoring of biliary output, supports the anastomosis, and allows access for cholangiography. Other than patient discomfort from prolonged presence of a percutaneous tube, the main limitation of T-tube placement is biliary leakage after its removal.
Shortage of donor organs, primarily in children awaiting a suitable liver for transplantation, has driven the development of reduced size, split liver, and living related donor transplantation techniques. These techniques increase the available supply of donor organs to younger recipients. Reduced size liver transplantation uses a portion of the liver as a graft but is an inefficient use of donor organs. Split liver transplantation, whereby the whole liver is used for transplantation for two recipients, is an attractive means of increasing the use of a limited supply of donor organs to both children and adults. Living related donor transplantation uses a portion of the donor liver. The lateral segment may be used successfully for small or pediatric patients with end-stage liver disease. A larger graft is needed for adult living donor transplantation, and whole right hepatic lobe grafts have been used successfully in many transplantation centers.
Living Donor Liver Transplantation
The number of cadaveric donors available for liver transplantation has remained constant for several years. As thousands of patients are on the waiting list, living donor liver transplantation (LDLT) can be a solution for these patients. However, LDLT requires balancing donor risk relative to the recipient outcome and has not become popular in the United States. Only 219 LDLTs were performed in the United States in 2009, probably because of the risk of donor morbidity and mortality. LDLT is more popular in countries with scarcity of deceased donors.
LDLT was initially performed in the 1990s for small or pediatric patients with end-stage liver disease. Procedures using the left lobe or lateral segment were performed at the University of Chicago. Although these techniques were attempted in adult recipients, the smaller left hepatic lobe provided insufficient hepatic mass for most adult patients. The first adult-to-adult right hepatic lobe transplantation was reported in 1993 by Yamaoka and colleagues. The first successful donor right hepatic lobe transplantation was performed in the United States in 1997.
Other than increasing the pool of potential donors, LDLT has some other advantages. Patients can undergo timely transplantation without waiting for a deceased donor organ. This may confer survival benefit to LDLT, which has been shown to be higher compared with deceased donor transplantation. A detailed preoperative assessment and clinical management of often critically ill potential recipients are possible. Finally, living donor grafts are obtained from healthy donors who have been extensively screened for disease. Despite these advantages, there are unique risks of LDLT. The risk of donor death is 1.7 per 1000 in the United States. Clavien grade 3 or 4 complications are seen in 2.8% of the donors.
Imaging Evaluation of the Potential Living Related Liver Donor
The ideal screening modality for potential living liver donors would accurately evaluate hepatic parenchyma, provide data for split liver volume assessment, and depict intrahepatic and extrahepatic vascular anatomy before potential partial hepatectomy. MRI is used to depict the vascular variants, hepatic volume issues, and fatty infiltration that may preclude right hepatic lobe donation. Conventional or hepatocyte-specific contrast agent–enhanced MRI can be performed to demonstrate problems with biliary anatomy. Hepatocyte- and biliary-specific contrast agents (e.g., gadobenate dimeglumine, gadoxetate disodium [Gd-EOB-DTPA], mangafodipir trisodium ) not only allow dynamic contrast-enhanced scanning but also are differentially taken up by functioning hepatocytes and excreted into the biliary tree. This allows excellent delineation of the biliary anatomy.
The exquisite spatial resolution of multidetector CT has made it the most widely used modality for the preoperative evaluation of living donors. Rapid scanning makes bolus timing even more critical; either test boluses or automated triggering is crucial for obtaining images during peak hepatic arterial enhancement. The 2.5-mm collimated images are then obtained during a portal venous (“hepatic”) phase so that portal and hepatic venous anatomy may be displayed. Both the source and reconstructed images should be meticulously reviewed. Volume rendered images are helpful for displaying global anatomy, but many radiologists rely heavily on sliding thick-slab maximum intensity images.
Parenchymal Assessment
The size of the potential right lobe graft is a crucial determinant of graft viability. A sufficiently large right lobe is needed to prevent the “small for size” syndrome; transplantation of insufficient hepatic mass may result in poor allograft function, graft failure, or the death of the recipient. Small grafts are also more prone to sinusoidal injury from increased portal pressures. Several approaches to assessment of sufficient liver volume for transplantation have been applied; graft weight/recipient standard liver volumes of less than 40% or graft-to-recipient weight ratios of less than 85% are associated with poor outcomes. Both MRI and CT can be used to accurately calculate split liver volumetric measurements of potential donors.
Unenhanced CT or in-phase/out-of-phase MRI is also used to detect fatty infiltration in the potential donor graft. It has been well shown that steatosis increases the likelihood of graft dysfunction and reduces hepatic regenerative function. Several centers still routinely perform liver donor biopsies to better quantify steatosis.
Vascular Assessment
Anatomic variations of extrahepatic vascular anatomy (e.g., replaced left or right hepatic arteries) are common, well known, and easily demonstrated by CT angiography. The unique contribution of multidetector CT to the evaluation of the prospective right liver donor is its ability to detect variations in arterial and venous anatomy that may traverse the hepatectomy plane. Although adequate hepatic mass may be procured with several techniques (left donor hepatectomy, extended right donor hepatectomy), a traditional right hepatectomy along Cantlie’s line, a relatively avascular plane immediately lateral to the middle hepatic vein, is preferred in most institutions. Parenchymal dissection to the hilar plate is performed by ultrasonic dissection. Accurate definition of vascular structures traversing this plane is needed to prevent inadvertent vessel injury and ischemic injury to either the graft or remaining donor liver.
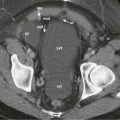
Traditional hepatic arterial anatomy, in which the proper hepatic artery branches into right and left hepatic branches, is seen in only 55% of patients. Although many variants are easily shown at CT, a crucial consideration for donor graft retrieval is the blood supply to segment IV. A middle hepatic artery branch that arises from the right hepatic artery will be disrupted during donor hepatectomy. Inadvertent ligation of this branch during graft retrieval may cause biliary ischemic damage to the remaining donor medial segment. Other significant vascular variants that may affect donor selection include a short right hepatic artery, trifurcation of the common hepatic artery into the gastroduodenal artery and right and left hepatic arteries, and origination of the right or left hepatic arteries before the gastroduodenal artery. Replaced hepatic arteries in the recipient are not absolute contraindications to transplantation, although arterial reconstructions can be more technically demanding. Finally, recipient celiac artery stenosis from atherosclerotic disease or a median arcuate ligament may predispose to graft infarction and biliary complications. Sliding thick-slab maximum intensity projection images obtained from a second portal venous (hepatic) phase are used to display potentially significant hepatic venous variants. Variations in branching of the middle hepatic vein are particularly important because they may necessitate an altered hepatectomy plane. For example, inadvertent ligation of a prominent early branch (>3 mm) of the middle hepatic vein draining segment VIII may result in segmental hepatic venous congestion in the transplanted liver ( Fig. 92-5 ). The early branch may be spared with a more lateral hepatectomy plane (although this may decrease the volume of the graft), or the surgeon may maintain outflow drainage to segment VIII by directly anastomosing the branch to the recipient IVC. Inferior accessory right hepatic veins are also common variants that may have an impact on graft retrieval; most transplant surgeons will try to preserve inferior accessory veins larger than 3 to 5 mm. If the distance between the vein and the hepatic venous confluence is less than 4 cm, it may be possible to implant both the main and accessory right veins with a single partially occluding clamp on the IVC.

Variations in portal venous anatomy also need to be considered before right hepatic lobe donation. Early right portal vein bifurcation or main portal vein trifurcation may make transplantation difficult. The portal vein may be reconstructed on the back table, and vein or interposition grafts may be employed, but this increases the risk of portal vein thrombosis ( Fig. 92-6 ). Portal venous flow to segment IV should also be carefully delineated; flow to the medial segment can arise from either the right or left portal vein; inadvertent disruption of portal venous inflow during donor hepatectomy may result in remaining donor liver ischemic injury. Flow to the entire left lobe through a branch from the right portal vein is an absolute contraindication to organ donation.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree