CHAPTER 3 Local Staging: Imaging Options and Core Biopsy Strategies
Treatment planning for newly diagnosed breast cancer patients, including local and systemic therapies, is based on tumor type, extent of disease, and accurate staging. Imaging and image-directed needle biopsies play a critical role in establishing the local extent of disease and aid in staging. Surgical decision making, between breast-conserving therapy (also termed lumpectomy or segmental or partial mastectomy) and mastectomy, is primarily based on tumor size, extent and location within the breast, cosmetic implications, and patient preference. Imaging modalities, including mammography, ultrasound, MRI, and molecular imaging, are used to determine the extent of disease. However, disease extent cannot be reliably established solely by imaging. When preoperative imaging suggests more extensive disease than clinical impressions, histologic confirmation is necessary before performing a more extensive surgery.
Imaging is used to locally stage breast cancer. The TNM stage is based on the size of the tumor and whether the cancer has spread (Table 1). Identification of abnormal adenopathy (axillary, supraclavicular, or internal mammary nodes), as well as involvement of the skin, pectoralis muscle, or chest wall, affects staging and therapeutic decision making. Options for local therapies include surgery and radiation therapy, whereas systemic treatments may include chemotherapy, hormone therapy, and biologic therapy. Imaging also is used to identify distant metastases.
Table 1 AMERICAN JOINT COMMITTEE ON CANCER STAGING SYSTEM FOR PATIENTS WITH BREAST CANCER
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| Tis (DCIS) | Ductal carcinoma in situ |
| Tis (LCIS) | Lobular carcinoma in situ |
| Tis (Paget’s) | Paget’s disease of the nipple with no tumor |
| TI | Tumor 2 cm or less in greatest dimension |
| T1mic | Microinvasion 0 to 1 cm or less in greatest dimension |
| T1a | 0.1 to 0.5 cm |
| T1b | >0.5 to 1 cm |
| T1c | >1 to 2 cm |
| T2 | Tumor >2 to 5 cm in greatest dimension |
| T3 | Tumor >5 cm in greatest dimension |
| T4 | Tumor of any size with direct extension to chest wall or skin |
| T4a | Extension to chest wall, not including pectoral muscle |
| T4b | Edema (including peau-d’orange) or ulceration of the skin of the breast, or satellite skin nodules confined to the same breast |
| T4c | T4a and T4b |
| T4d | Inflammatory carcinoma |
| Regional Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed (e.g., previously removed) |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in movable ipsilateral axillary lymph nodes |
| N2 | Metastasis in ipsilateral axillary lymph nodes fixed or matted, or in clinically apparent ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastasis. |
| N2a | Metastasis in ipsilateral axillary lymph nodes fixed to one another or to other structures |
| N2b | Metastasis only in clinically apparent ipsilateral internal mammary nodes and in the absence of clinically evident axillary lymph node metastasis |
| N3 | Metastasis in ipsilateral infraclavicular lymph node(s) with or without axillary lymph node involvement, or in clinically apparent ipsilateral internal mammary node(s) in the presence of clinically evident axillary lymph node metastasis; or metastasis in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement |
| N3a | Metastasis in ipsilateral infraclavicular lymph node(s) and axillary lymph node(s) |
| N3b | Metastasis in ipsilateral internal mammary lymph node(s) nodes and axillary lymph node(s) |
| N3c | Metastasis in ipsilateral supraclavicular lymph node(s) |
| Distant Metastasis (M) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
EXTENT OF DISEASE
Most breast cancers are evaluated by mammography, ultrasound, or both modalities. It is important to document the size, location, and distribution of the primary lesion, but also to evaluate for satellite lesions. Preoperative identification of additional lesions improves the likelihood of obtaining clear margins if breast-conserving therapy (BCT) is performed. For masses, it is important to look for associated microcalcifications, which may represent an associated noninvasive (in situ) component. The term extensive intraductal component (EIC) is used to refer to invasive tumors in which ductal carcinoma in situ (DCIS) makes up at least 25% of the neoplasm. Of all invasive ductal carcinomas, 15% to 30% have an EIC.1 Tumors that are predominantly DCIS with focal invasion are also classified as EIC. The presence of EIC may have prognostic implications on the likelihood of obtaining clear margins, as well as the risk for subsequent local recurrence.2,3
As many as 30% to 60% of breast cancers are pathologically multifocal (more than one tumor focus, separated by normal tissue) at the time of diagnosis.4,5 The term multicentric has been variably defined. It generally has been used to describe cancers separated by more than 4 cm or tumors located in different quadrants of the breast. BCT generally is not suitable for multicentric carcinomas because of poor cosmetic results, limitations of radiation therapy, and inability to obtain clear margins.
Synchronous contralateral breast cancer may occur in about 3% to 5% of women with breast cancer.6,7 Identification of these lesions at the time of the contralateral index cancer diagnosis can facilitate treatment in a single surgery, thereby avoiding both delays in diagnosis, as well as the emotional stress of a later diagnosis and second surgery.
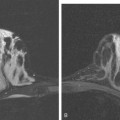
MRI is useful to identify residual disease and direct re-excision in patients with positive margins at initial lumpectomy (Figure 1).
EVALUATING ADENOPATHY
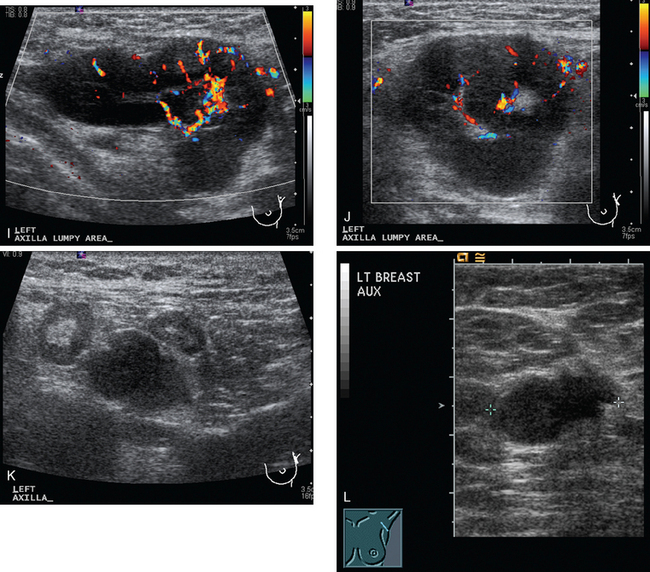
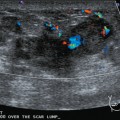
Identification of abnormal adenopathy, including axillary, supraclavicular, and internal mammary nodes, is important in staging. Metastatic adenopathy is suspected on imaging when there is cortical thickening (generally >3 mm), loss of the fatty hilum, and enlargement, particularly with increasingly round shapes8 (Figure 2 and Figure 3). However, because many benign processes may cause reactive nodes with similar imaging findings, fine-needle aspiration (FNA) or core needle biopsy is necessary to confirm suspected metastatic nodal disease. Conversely, the absence of suspicious imaging findings, whether on mammography, ultrasound, MRI, or molecular imaging studies, does not exclude metastatic nodal involvement, particularly for micrometastasis. Therefore, in addition to imaging, sampling, either with axillary dissection or sentinel lymph node biopsy, is essential in the staging of breast cancer patients.
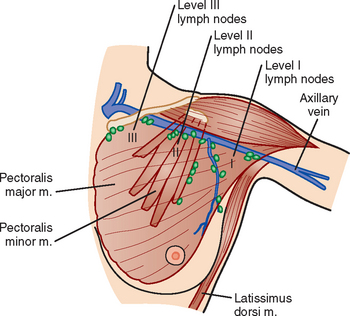
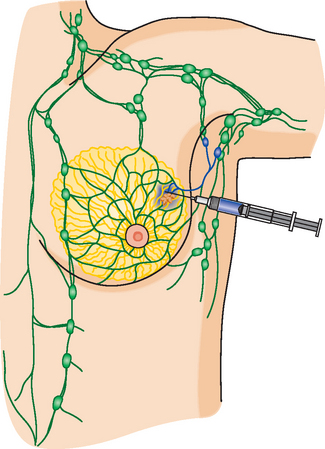
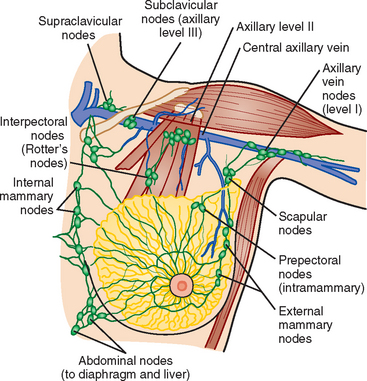
The axillary nodes form a chain from the underarm to the collarbone (Figure 4). The axillary lymph nodes are named in relation to the pectoralis minor muscle, with level I the lowest, lateral to the pectoralis minor muscle. Level I receives the most lymphatic drainage from the breast. Level II axillary nodes are beneath the pectoralis minor muscle. Level III is above and medial to the pectoralis minor muscle. A traditional axillary lymph node dissection usually removes nodes in levels I and II. Sentinel lymph node sampling involves the mapping and removal of the first lymph node or nodes (usually 1 to 3) that drain the involved area of the breast (Figure 5 and Figure 6). Instead of removing 10 or more lymph nodes as performed in a standard dissection, the status of the axilla can be predicted by excision and close pathologic examination of the sentinel node. Sentinel lymph node biopsy has significantly reduced the number of women undergoing standard axillary dissection, avoiding dissection-associated side effects such as arm lymphedema. The identification of abnormal lymph nodes on physical exam or imaging studies favors proceeding directly to axillary dissection over sentinel node biopsy.
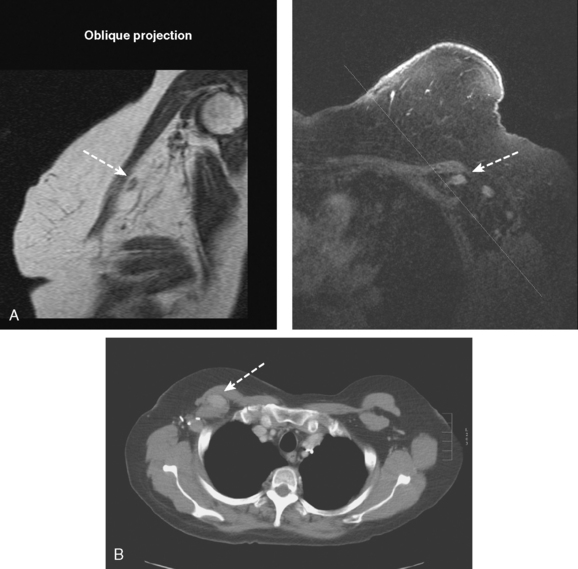
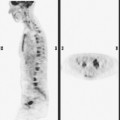
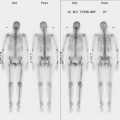
The use of molecular imaging studies, CT, or MRI may identify adenopathy in areas other than the axilla (Figure 7). The presence of internal mammary node adenopathy (Figure 8) affects staging and radiation therapy planning. Identification of abnormal Rotter’s nodes (Figure 9), nodes between the pectoralis minor and major muscles, also has staging and therapeutic implications.
SKIN, PECTORALIS, AND CHEST WALL INVOLVEMENT
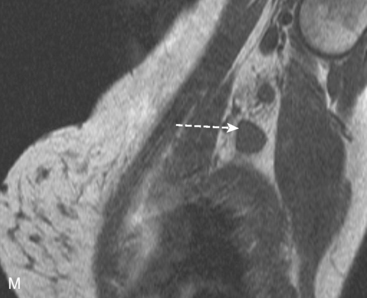
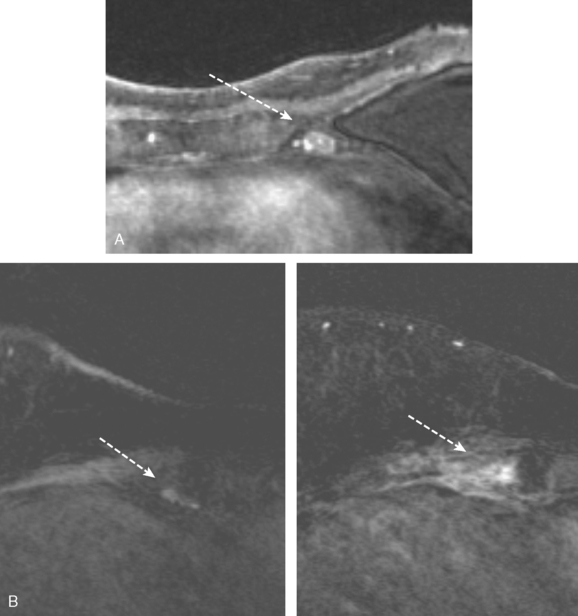
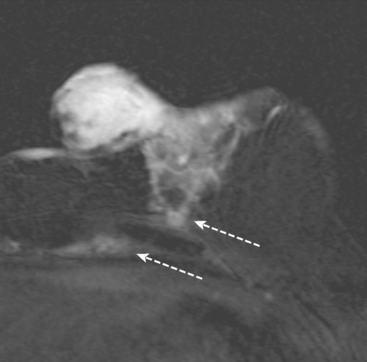
Identification of breast edema and skin thickening in patients with invasive breast cancer may represent an inflammatory component (tumor involving the dermal lymphatics). This materially affects staging and therapeutic approach. Skin punch biopsy may be necessary to confirm the diagnosis if the clinical picture is not characteristic. Identification of pectoralis muscle or chest wall involvement also affects treatment planning. Pectoralis muscle involvement should be looked for in women with posterior lesions. The diagnosis on MRI requires not just effacement or obliteration of the pectoralis fascia but also enhancement of the muscle (Figure 10). Identification of either skin or chest wall involvement classifies a tumor as a locally advanced breast carcinoma (LABC). Large (>5 cm) tumors and those with clinically matted or fixed axillary node involvement or involved supraclavicular or internal mammary nodes by imaging are also considered locally advanced. LABC is generally treated with preoperative (neoadjuvant or primary systemic) chemotherapy, which converts some patients into operative candidates. Multiple examples are presented in Chapter 5.
PREOPERATIVE MAGNETIC RESONANCE IMAGING
MRI is becoming increasingly established as a useful modality in patients with known breast cancer. However, patterns of use of preoperative MRI in women with biopsy-proven breast cancer remain highly variable in practice because of the lack of randomized control trials. It has been firmly established that preoperative MRI can detect unsuspected disease and often changes management in women with known breast cancer.9 However, the use of preoperative MRI to alter surgical management has been criticized because of the lack of studies evaluating its effect on tumor recurrence and mortality.10–12 Fischer and colleagues,13 in a study involving more than 40 months of follow-up, reported a reduction in ipsilateral breast tumor recurrence, from 6.8% to 1.2%, in patients who underwent preoperative MRI. However, this study was a retrospective, singleinstitution review of only 346 patients. It has been argued that unsuspected disease detected on MRI and treated with BCT is of little clinical consequence and is controlled by radiation therapy. This argument is primarily based on the fact that 10-year local recurrence rates as low as 10% have been reported in patients with negative surgical margins.14–16 Concerns about the use of preoperative MRI involve the potential for unnecessary biopsies, delays in treatment, and an increase in unnecessary mastectomies. Despite these arguments, there is ongoing evidence and experience accumulating that supports its benefit in preoperative staging, as follows:
FUNCTIONAL (MOLECULAR) BREAST IMAGING: BREAST-SPECIFIC GAMMA IMAGING AND POSITRON EMISSION MAMMOGRAPHY
Functional breast imaging is a growing and evolving field that is assuming a larger role in breast cancer diagnosis, providing complementary information to anatomically based breast imaging modalities. Scintimammography has matured from initial versions using standard gamma cameras, which were limited in resolution and positioning flexibility, to breast-specific gamma imaging (BSGI), which obtains higher-resolution planar images in views emulating mammography.21–25 Similarly, positron emission mammography (PEM) is performed using a small-field-of-view, high-resolution PET scanner that resembles a mammogram unit and acquires tomographic data sets in planes analogous to mammography.26–29 Scintimammography is performed after the intravenous administration of 25 mCi of either 99mTc-sestamibi or 99mTc-tetrofosmin, whereas PEM is performed after an hour’s uptake of 10 mCi of 18F-fluorodeoxyglucose (FDG) admin-istered intravenously. The radiation dose is about the same, about 0.4 rad. Imaging time is also similar. Scintimammography planar views are acquired for 5 to 10 minutes, or at least 150,000 counts, whereas PEM tomographic data sets are acquired for 4 to 10 minutes per projection. The lower limit of BSGI detector resolution is 3 mm (although smaller lesions may be identifiable), whereas the in plane resolution of PEM is on the order of 2 mm (Table 2).
Table 2 FUNCTIONAL BREAST IMAGING COMPARISON CHART
| BSGI | PEM | |
|---|---|---|
| Radiopharmaceutical | 18F-FDG (2-[fluorine-18] fluoro-2-deoxy-d-glucose | |
| Half-life | 6 hr | 110 min |
| Emission energy | 140 keV | 511 keV |
| Dose | 25 mCi | 10 mCi |
| Sensitivity | 91% | |
| Specificity | 87%–89% | 93% |
| Whole-body dosimetry | About 0.4 rad | About 0.4 rad |
| Acquisition | Planar | Tomographic |
| Binding target | Intracellular mitochondria | Intracellularly phosphorylated by hexokinase |
| Theoretical basis | Cancer cells have greater cytoplasmic mitochondrial density than normal breast | Higher glycolytic rate of cancer cells results in increased cellular uptake and glucose utilization |
| Negative predictive value (NPV) | 88% | |
| Target population | Suspicion of breast abnormality (breast cancer diagnosis not required) | Approved for patients with known or past history of breast cancer |
| Mechanism of cellular transport | Passive diffusion through potassium channels | Active transport into cell |
| Lower limit of resolution | 3-mm detector spatial resolution | 2 mm |
BSGI, breast-specific gamma imaging; PEM, positron emission mammography.
At this writing, BSGI is becoming more available, with sites with early experience finding it useful both for problem solving of ambiguous conventional breast imaging findings, and as an adjunct to local staging, looking for multifocality, multicentricity, and contralateral lesions (Figure 11). BSGI does not require a diagnosis of breast cancer for reimbursement, and increasingly is being performed in patients with suspicious conventional breast imaging findings as an aid to biopsy decision making (e.g., deciding how many areas need sampling). Currently, PEM is approved for patients with known or prior breast cancer diagnoses. Several cases incorporating the use of PEM have been included in this chapter. Its performance compared with MRI in preoperative local staging of apparently localized breast cancer is being assessed by a multicenter, prospective clinical trial, which at this writing is accruing patients. Early pilot studies show high sensitivity for depiction of primary breast cancers, on the order of 91%. Similarly high sensitivity for primary tumor depiction is reported for BSGI. Both modes of functional breast imaging appear to have improved specificity compared with MRI, on the order of 87% to 89% for BSGI and 93% for PEM, offering hope that increased use of functional breast imaging in the future may decrease the number of unnecessary benign biopsies now being performed.
1 van Dongen JA, Fentiman IS, Harris JR, et al. In situ breast cancer: the EORTC consensus meeting. Lancet. 1989;2:25-27.
2 Schnitt SJ, Connolly JL, Recht A, et al. Breast relapse following primary radiation therapy for early breast cancer. II. Detection, pathologic features and prognostic significance. Int J Radiat Oncol Biol Phys. 1985;11:1277-1284.
3 Bartelink H, Borger JH, van Dongen JA, Peterse JL. The impact of tumor size and histology on local control after breast conserving treatment. Radiother Oncol. 1988;11:297-303.
4 Holland R, Veling SH, Mravunac M, Hendricks JH. Histologic multifocality of Tis, T1–2 breast carcinomas: implications for clinical trials of breast conserving surgery. Cancer. 1985;56:979-990.
5 Wilkinson LS, Given-Wilson R, Hall T, et al. Increasing the diagnosis of multifocal primary breast cancer by the use of bilateral whole-breast ultrasound. Clin Radiol. 2005;60:573-578.
6 Heron DE, Komarnicky LT, Hyslop T, et al. Bilateral breast carcinoma: risk factors and outcomes for patients with synchronous and metachronous disease. Cancer. 2000;88:2739-2750.
7 Liberman L, Morris EA, Kim CM, et al. MR imaging findings in the contralateral breast of women with recently diagnosed breast cancer. AJR Am J Roentgenol. 2003;180:333-341.
8 Yang WT, Chang J, Metreweli C. Patients with breast cancer: differences in color Doppler flow and gray-scale US features of benign and malignant axillary lymph nodes. Radiology. 2000;215:568-573.
9 Bedrosian I, Mick R, Orel SG, et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer. 2003;98:468-473.
10 Morrow M. Magnetic resonance imaging in the preoperative evaluation of breast cancer: primum non nocere. J Am Coll Surg. 2004;198:240-241.
11 Morrow M. Magnetic resonance imaging in breast cancer: one step forward, two steps back? JAMA. 2004;292:2779-2780.
12 Morrow M. Magnetic resonance imaging in breast cancer: is seeing always believing? Eur J Cancer. 2005;41:1368-1369.
13 Fischer U, Zachariae O, Baum F, et al. The influence of preoperative MRI of the breasts on recurrence rate in patients with breast cancer. Eur Radiol. 2004;14:1725-1731.
14 Smitt MC, Nowels KW, Zdeblick MJ, et al. The importance of the lumpectomy surgical margin status in long-term results of breast conservation. Cancer. 1995;76:259-267.
15 Neuschatz AC, DiPetrillo T, Safaii H, et al. Long-term follow-up of a prospective policy of margin-directed radiation dose escalation in breast-conserving therapy. Cancer. 2003;97:30-39.
16 Obedian E, Haffty BG. Negative margin status improves local control in conservatively managed breast cancer patients. Cancer J Sci Am. 2000;6:28-33.
17 Provenzano E, Hopper JL, Giles GG, et al. Histological markers that predict clinical recurrence in ductal carcinoma in situ of the breast: an Australian population-based study. Pathology. 2004;36:221-229.
18 Borg MF. Breast-conserving therapy in young women with invasive carcinoma of the breast. Australas Radiol. 2004;48:376-382.
19 Smitt MCMD, Horst K. Association of clinical and pathologic variables with lumpectomy surgical margin status after preoperative diagnosis or excisional biopsy of invasive breast cancer. Ann Surg Oncol. 2007;14(3):1040-1044.
20 Lehman CD, Gatsonis C, Kuhl CK, et al. ACRIN Trial 6667 Investigators Group. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356(13):1295-1303.
21 Schillaci O, Buscombe JR. Breast scintigraphy today: indications and limitations. Eur J Nucl Med Mol Imaging. 2004;31(Suppl 1):S35-S45.
22 Brem RF, Schoonjans JM, Kieper DA, et al. High-resolution scintimammography: a pilot study. J Nucl Med. 2002;43:909-915.
23 Brem RF, Rapelyea JA, Zisman G, et al. Occult breast cancer: scintimammography with high-resolution breast-specific gamma camera in women at high risk for breast cancer. Radiology. 2005;237:274-280.
24 Brem RF, Fishman M, Rapelyea JA. Detection of ductal carcinoma in situ with mammography, breast specific gamma imaging, and magnetic resonance imaging: a comparative study. Acad Radiol. 2007;14(8):945-950.
25 Brem RF, Petrovitch I, Rapelyea JA, et al. Breast-specific gamma imaging with (99m) Tc-sestamibi and magnetic resonance imaging in the diagnosis of breast cancer: a comparative study. Breast J. 2007;13(5):465-469.
26 Levine EA, Freimanis RI, Perrier ND, et al. Positron emission mammography: initial clinical results. Ann Surg Oncol. 2003;10:86-91.
27 Rosen EL, Turkington TG, Soo MS, et al. Detection of primary breast carcinoma with a dedicated, large-field-of-view FDG pet mammography device: initial experience. Radiology. 2005;234:527-534.
28 Berg WA, Weinberg IN, Narayanan D, et al. High-resolution fluorodeoxyglucose positron emission tomography with compression (“positron emission mammography”) is highly accurate in depicting primary breast cancer. Breast J. 2006;12(4):309-323.
29 Tafra L, Cheng Z, Uddo J, et al. Pilot clinical trial of 18F-fluorodeoxyglucose positron-emission mammography in the surgical management of breast cancer. Am J Surg. 2005;190(4):628-632.
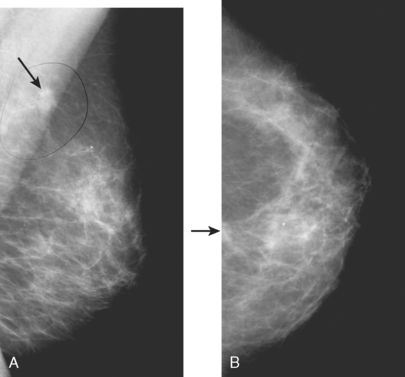
CASE 1 Mammography: Extent of disease
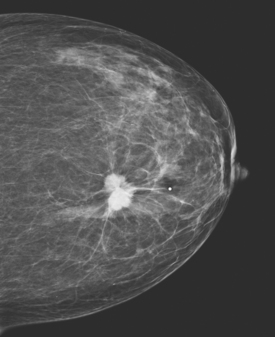
A 75-year-old woman presented with a palpable left breast mass. Mammography demonstrated a dense, spiculated breast cancer, corresponding to the palpable mass (Figure 1). Closer review of the mammogram showed a second abnormality distant from the palpable mass, with suspicious linear microcalcifications in the central left breast (Figure 2). Biopsy of the mass revealed invasive ductal carcinoma and stereotactic biopsy of the calcification found intermediate-grade ductal carcinoma in situ (DCIS). Because the two areas of disease involved a large portion of the left breast, mastectomy was performed.
Fisher B, Anderson S, Bryant J, et al. Twenty year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233-1241.
Holland R, Veling SH, Mravunac M, Hendriks JH. Histologic multifocality of Tis, T1–2 breast carcinomas: implications for clinical trials of breast-conserving surgery. Cancer. 1985;56(5):979-990.
Veronesi U, Cascinelli N, Mariani L, et al. Twenty year follow-up of a randomized study comparing breast conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227-1232.
Wazer DE, Schmidt-Ullrich RK, Schmid CH, et al. The value of breast lumpectomy margin assessment as a predictor of residual tumor burden. Int J Radiat Oncol Biol Phys. 1997;38(2):291-299.
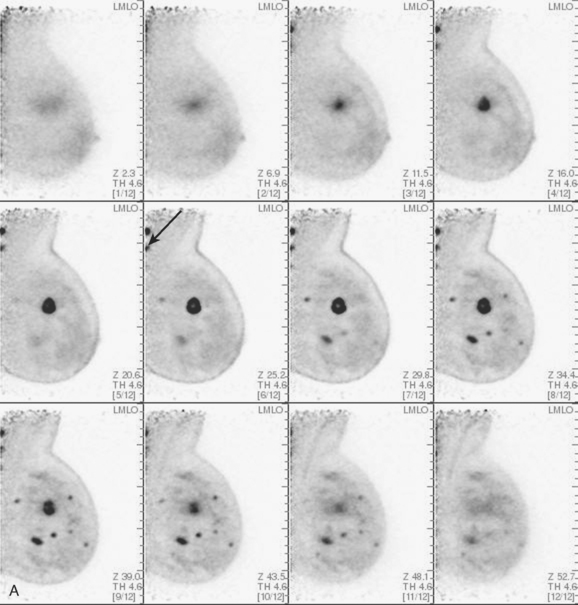
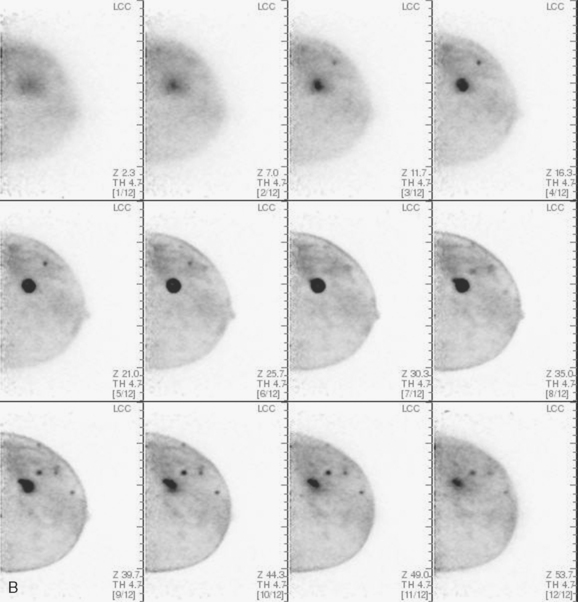
CASE 2 Use of ultrasound to find invasive disease within extensive microcalcifications; depiction of disease extent by breast MRI versus PEM versus whole-body PET
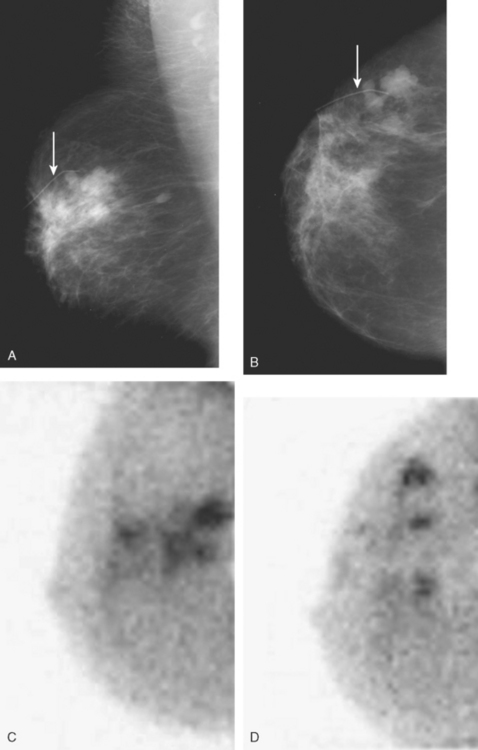
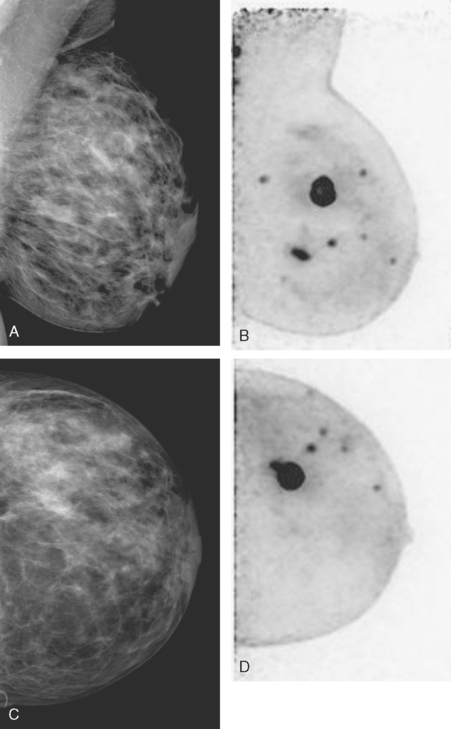
An asymptomatic 40-year-old woman had extensive new left lateral microcalcifications identified on screening mammography. These showed suspicious linear and branching pleomorphism on magnification views (Figure 1), and extensive ductal carcinoma in situ (DCIS) was suspected. Ultrasound was performed to determine whether an invasive component could be identified for biopsy. Five solid masses were identified in the left lateral breast, ranging up to 1.8 cm in size, as well as a suspicious abnormal axillary lymph node (Figure 2).
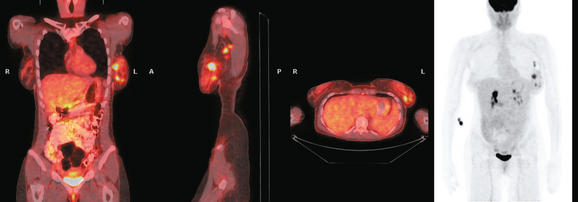
The patient desired breast conservation, and so extensive staging studies were performed to assess the extent of disease. These included breast MRI (Figure 3), positron emission mammography (PEM) (Figure 4 and Figure 5), and whole-body positron emission tomography (PET) (Figure 6). These studies confirmed multicentric disease with axillary nodal involvement, but showed no distant metastases. Neoadjuvant chemotherapy was given.
TEACHING POINTS
Both invasive and noninvasive disease components are well depicted by MRI. The DCIS manifested as confluent, segmental enhancement in the lateral breast. Eight intensely enhancing discrete masses with washout could be identified by MRI within this extensive intraductal component. PEM performed nearly as well, depicting seven discrete sites of intense increased fluorodeoxyglucose (FDG) uptake. The intraductal disease (as represented by the distribution of calcifications on mammography) would be difficult to recognize prospectively on PEM, without mammographic correlation. The lower-level segmental FDG uptake seen here in the distribution of the DCIS calcifications also corresponds to the distribution of parenchymal density and so would be difficult to differentiate from background parenchymal uptake.
CASE 3 MRI: Extent of disease
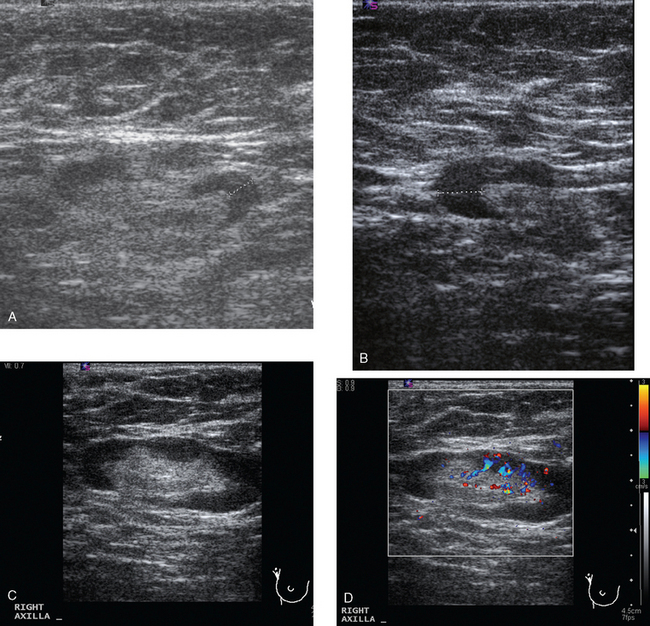
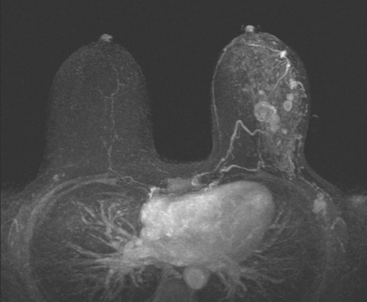
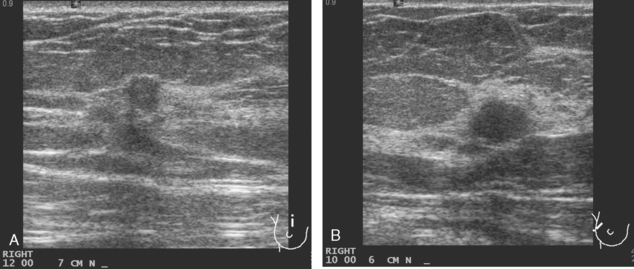
A 54-year-old woman presented with a palpable mass in the upper outer left breast. Diagnostic mammographic and ultrasound evaluations demonstrated multiple masses in the upper outer quadrant of the left breast at the site of the palpable abnormality (Figure 1 and Figure 2). Core needle biopsy confirmed invasive ductal carcinoma. Preoperative MRI suggested much more extensive involvement of the left breast, with multiple enhancing masses extending from area of the known cancer toward the nipple (Figure 3). Because of the MRI findings, second-look ultrasound was performed and identified several small masses in the subareolar region (Figure 4). Core needle biopsy confirmed the extensive nature of the patient’s disease, and she was treated surgically with mastectomy and axillary dissection.
CASE 4 Multicentric IDC and DCIS: Local staging with MRI
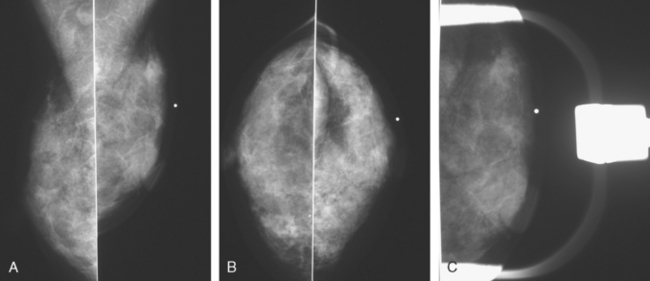
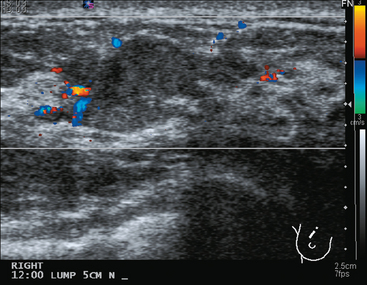
Mammography showed very dense breast tissue, with no correlate for the palpable abnormality (Figure 1). Ultrasound of the palpable lump showed a 2-cm heterogeneous, solid mass, with irregular margins and vascularity and a highly suspicious appearance (Figure 2). An ultrasound-guided core needle biopsy confirmed infiltrating ductal carcinoma (IDC), with high-grade ductal carcinoma in situ (DCIS). At initial surgical consultation, breast conservation therapy with partial mastectomy and radiation was discussed. Because of the mammographic density of the patient’s breasts, the patient was referred for preoperative breast MRI to more fully evaluate her suitability for breast conservation therapy.
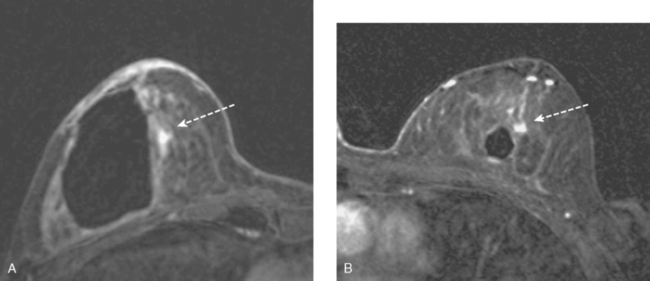
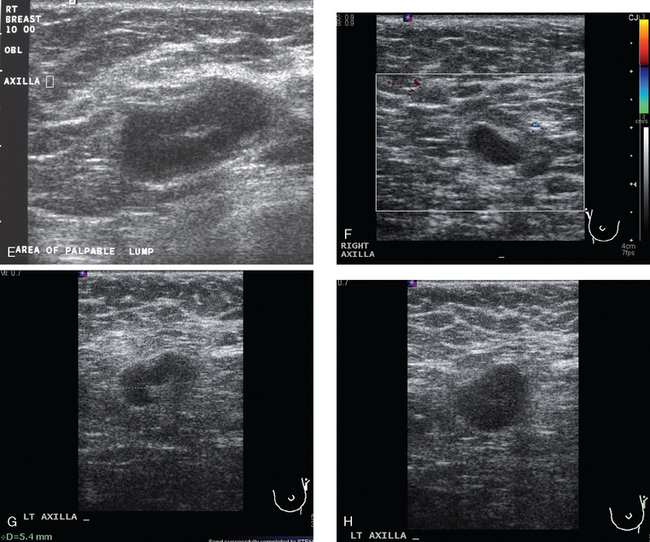
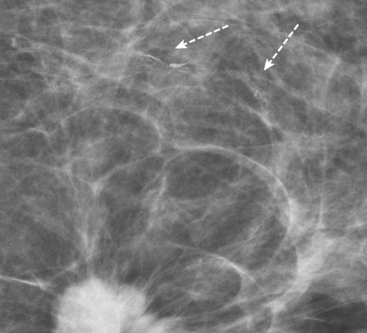
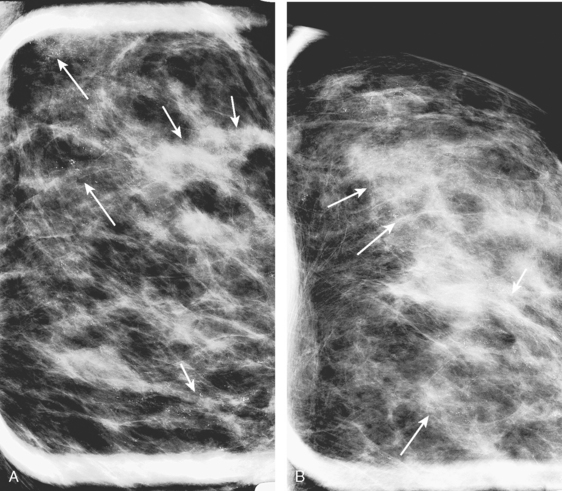
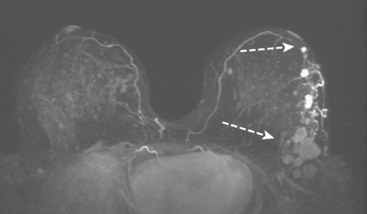
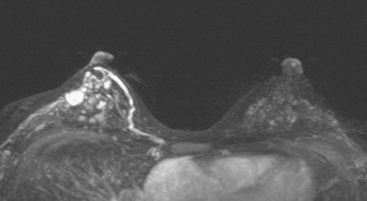
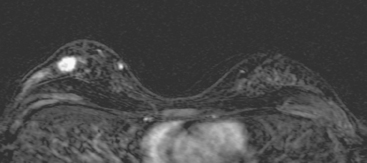
Bilateral enhanced subtracted breast MRI showed the known right breast carcinoma mass to be intensely enhancing, with irregular margins and spiculation. Multiple smaller, additional foci of enhancement were noted throughout the right breast, markedly asymmetric compared with the left side (Figure 3 and Figure 4). A few of these foci were larger and more morphologically concerning, including an irregular mass at 5 o’clock (Figure 5) and clumped contiguous foci of enhancement at 9 o’clock (Figure 6). A second-look ultrasound was performed of the right breast seeking correlates for biopsy, to prove the patient’s disease was multicentric and that she was not a conservation candidate.
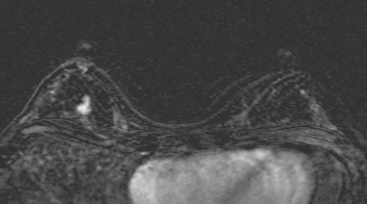
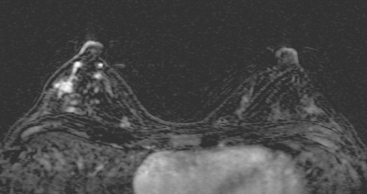
FIGURE 5 Another enhancing, irregularly bordered mass is seen just below the level of the nipple (partially visualized here). This was reported as being at 12 o’clock. However, the sonographic correlate (see Figure 7) was best seen at 5 o’clock. This discrepancy illustrates the difficulty in lesion localization between modalities, which is due both to the mobility of breast tissue and differences in positioning. This patient had very small breasts, and this is a centrally positioned lesion.
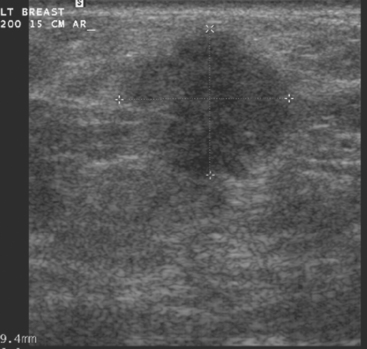
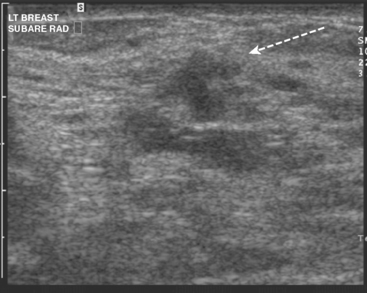
Ultrasound identified two subtle correlates for biopsy, confirming DCIS at both sites (Figure 7 and Figure 8). With pathologic confirmation of multicentric disease, the surgery treatment was changed to mastectomy.
TEACHING POINTS
This case illustrates well how a multimodality approach can yield critical additional information to most accurately stage a newly identified breast cancer. Mammography was not particularly useful in this patient, owing to breast density, but confirmed that there were no microcalcifications. Prospectively, ultrasound identified the index tumor and provided guidance to confirm the diagnosis of IDC. However, no additional disease sites were recognized prospectively on ultrasound to suggest multicentric disease. However, with the breast MRI as a roadmap for a directed, second-look ultrasound, more subtle correlates could be discerned and targeted for sampling, confirming two separate sites of DCIS remote from the IDC, indicating that the patient’s disease should be treated surgically with mastectomy.
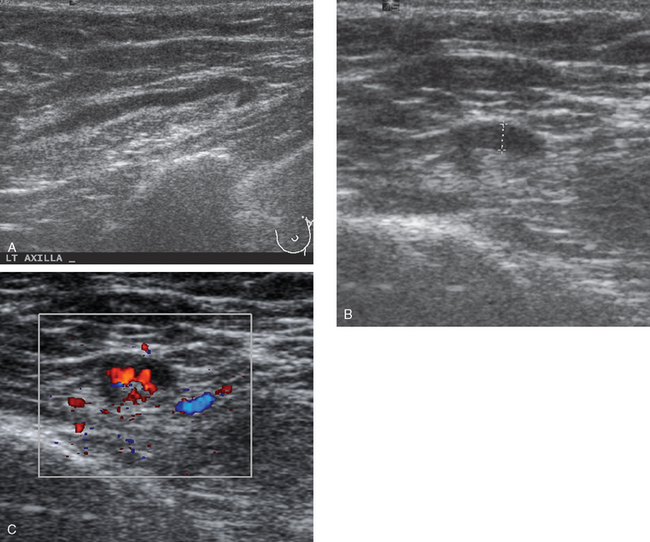
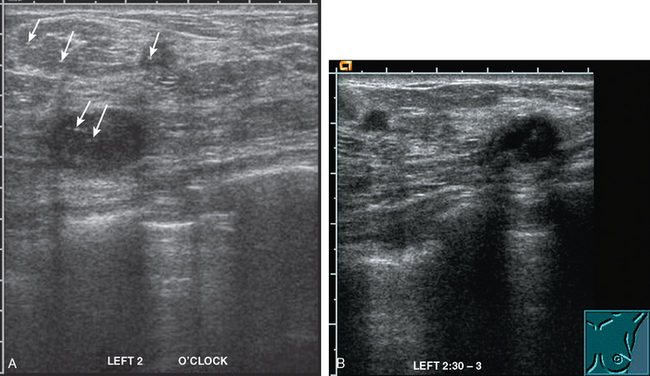
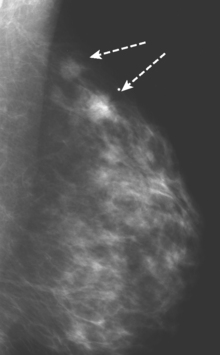
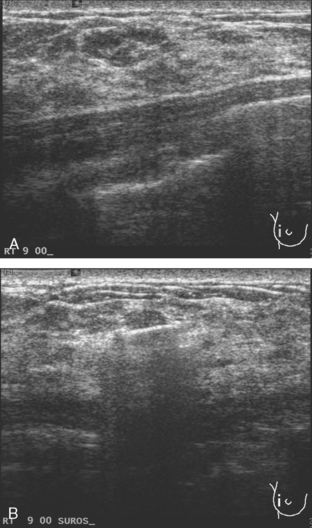
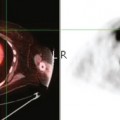
CASE 5 Additional disease site identified by PEM
A 46-year-old woman was noted to have a suspicious 1-cm spiculated mass overlying the right pectoral muscle on baseline screening mammography (Figure 1). It was confirmed on spot compression views, and a suspicious sonographic correlate was found (Figure 2), as well as a second concerning ultrasound finding. Both were biopsied with ultrasound guidance, confirming infiltrating ductal carcinoma (IDC) at 12 o’clock and sclerosing adenosis at 10 o’clock.