CHAPTER 5 Locally Advanced Breast Cancer (LABC) and Neoadjuvant Chemotherapy
DEFINITION OF LOCALLY ADVANCED BREAST CANCER
The definition of locally advanced breast cancer (LABC) has continued to evolve since Haagensen and Stout outlined their criteria for operability more than 60 years ago.1 Their conclusions remain a part of the current American Joint Committee on Cancer Classification System for LABC, as depicted in Table 1. LABC includes large tumors (>5 cm), those (of any size) with involvement of the skin or chest wall, and those with clinically apparent axillary nodal involvement (matted or fixed) or ipsilateral internal mammary, infraclavicular, or supraclavicular nodal disease.
Table 1 Primary Tumor (T) and Clinical Regional Lymph Node (N) Categories Comprising LABC in the Current American Joint Committee on Cancer Classification System
| T3 | Tumor >5 cm in greatest diameter |
| T4 | Tumor of any size, with direct extension to chest wall or skin, as described below |
| T4a | Extension to chest wall, not including pectoralis muscle |
| T4b | Edema (including peau d’orange) or ulceration of the skin of the breast or satellite nodules confined to same breast |
| T4c | Both Ta and Tb |
| T4d | Inflammatory carcinoma |
| N2 | Metastases in ipsilateral axillary nodes fixed or matted, or in clinically apparent ipsilateral internal mammary nodes in the absence of clinically evident axillary node metastases. |
| N3 | Metastases in ipsilateral infraclavicular or supraclavicular lymph nodes or in clinically apparent internal mammary nodes in the presence of clinically evident axillary node metastases. |
Data from Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual, 6th ed. New York, Springer Verlag, 2002.
ASSESSMENT OF PATIENTS SUSPECTED TO HAVE LABC
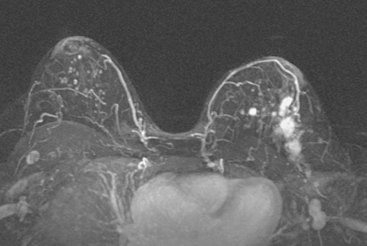
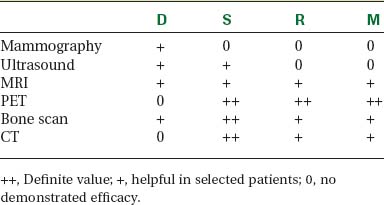
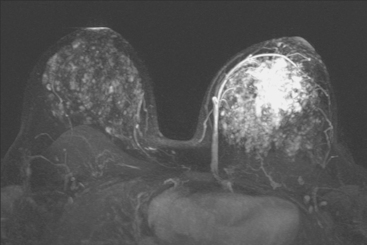
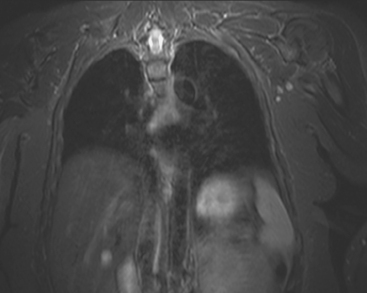
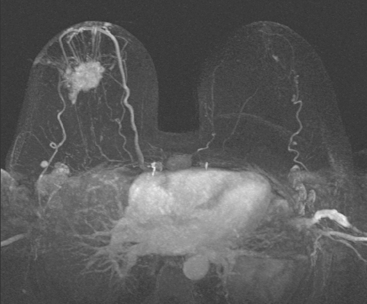
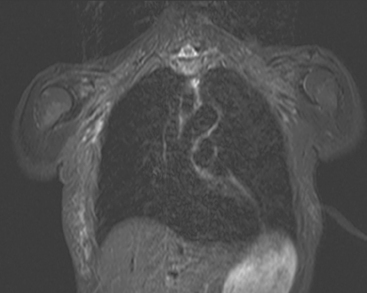
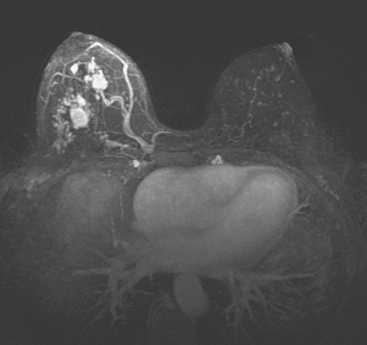
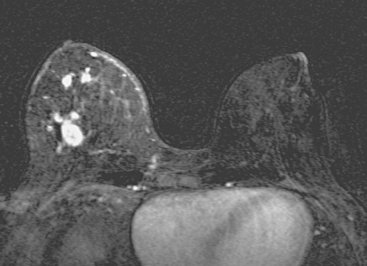
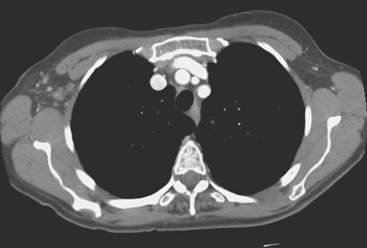
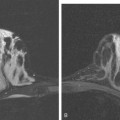
Locoregional staging of breast cancer is covered in depth in Chapter 3, including assessment with mammography, ultrasound, and MRI. The principles outlined in that chapter are identical for the patient who meets the definition of LABC. MRI has proved extremely valuable in accurately estimating tumor size and extent, as well as determining multifocality, multicentricity, and bilaterality (Figure 1). MRI more accurately correlates with tumor size and number of tumors at pathology than mammography or ultrasound. It is the modality of choice for assessment of chest wall invasion (Figure 2).
ASSESSMENT OF RESPONSE TO NEOADJUVANT CHEMOTHERAPY
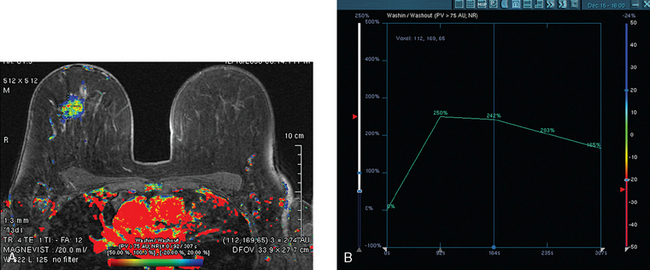
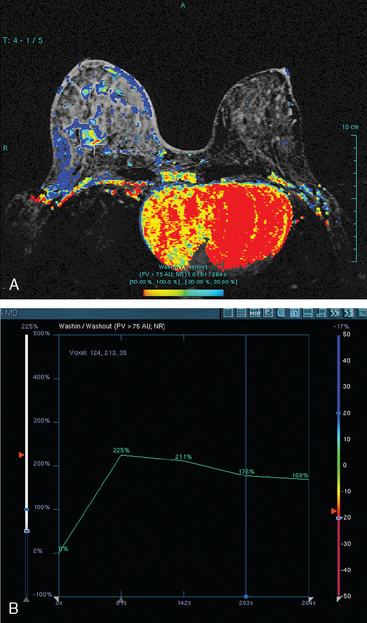
Assessment of response to neoadjuvant chemotherapy with anatomic imaging modalities can be problematic. On mammography, breast cancers that initially manifest as masses generally decrease in size in response to chemotherapy, but may not completely resolve. Breast cancer manifesting on mammography as microcalcifications is even more difficult to accurately assess in terms of responsiveness to neoadjuvant therapy because microcalcifications may not resolve, even in responders. On ultrasound, responding tumors show a decrease in size and, occasionally, resolution. MRI appears to be the most reliable anatomic imaging modality in common use today for monitoring patients being treated preoperatively.3–8 In addition to showing decreased tumor size, the enhancement pattern changes in responders. Responding tumors showing intense enhancement and washout initially often show decreased intensity of peak enhancement, as well as more benign patterns of progressive or persistent enhancement (flattening of the enhancement curve). Responding tumors may shrink, retaining a smaller, but still mass-like, morphology, or they can “break apart,” manifesting as less intense, smaller foci of enhancement within the distribution of the initial abnormality. Complete response by MRI (complete resolution of all tumor-associated enhancement) does not confirm a complete pathologic response because some patients with complete imaging responses have microscopic disease at pathology. Conversely, some patients with good, but incomplete, responses by MRI (some residual enhancement in the distribution of the original tumor) prove at pathology to have had complete pathologic responses.
PET and PET/CT are assuming an increasing role in this assessment, and a larger role for breast-specific gamma imaging and positron emission mammography can be anticipated in the future. Quantitative PET assessment, using the standardized uptake value (SUV), has shown early success in separating responders from nonresponders.10–12 Careful attention must be paid to all the factors influencing SUV measurement, especially region-of-interest assignment and patient glucose level. Flare responses (increased uptake as a manifestation of response), which can be seen on PET with initiation of tamoxifen therapy, are not seen with chemotherapy.
ASSESSMENT OF SENTINEL NODE STATUS IN LABC TREATED NEOADJUVANTLY
A controversial issue in the use of neoadjuvant chemotherapy is whether to assess preoperatively the status of the axilla with sentinel lymph node (SLN) sampling. It has been demonstrated that information from SLN biopsy or axillary dissection performed after completion of chemotherapy correlates well with overall prognosis. The role of SLN sampling and axillary dissection in the postchemotherapy setting was reviewed in a meta-analysis, with an overall accuracy of 95%.13
ASSESSMENT FOR SYSTEMIC METASTASES (EXCLUSION OF STAGE IV DISEASE)
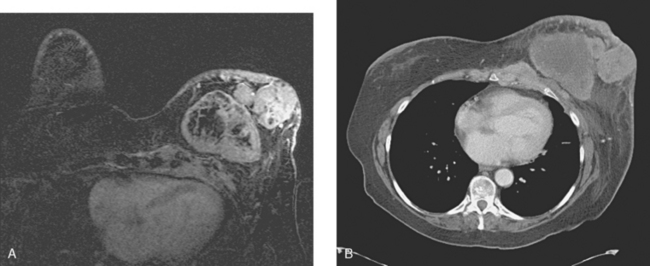
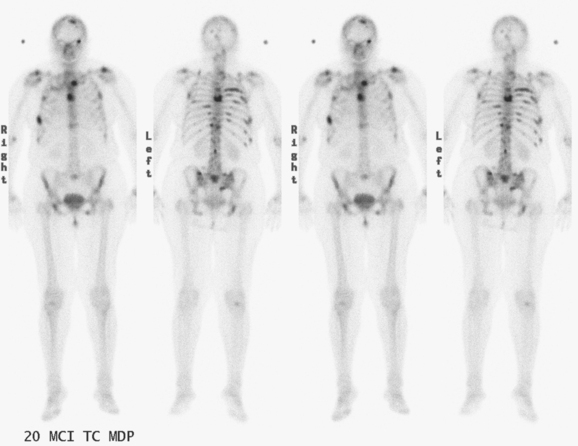
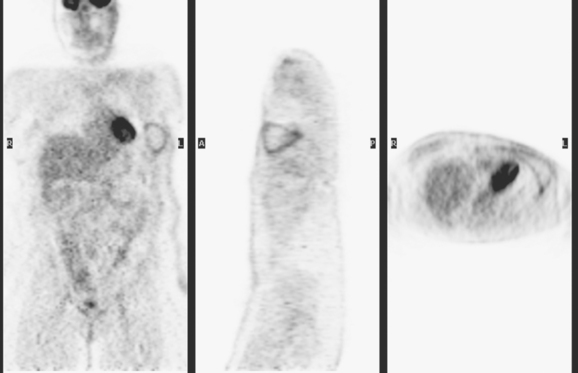
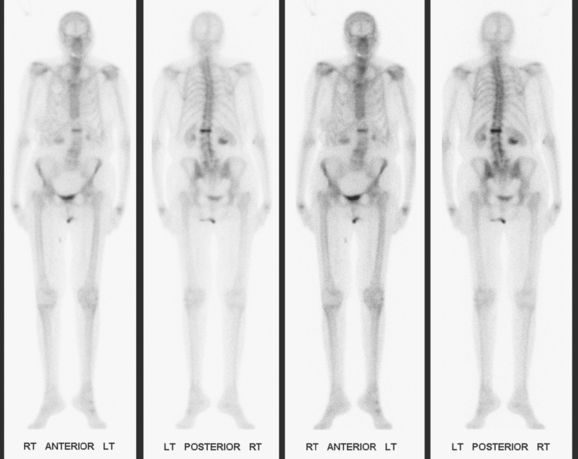
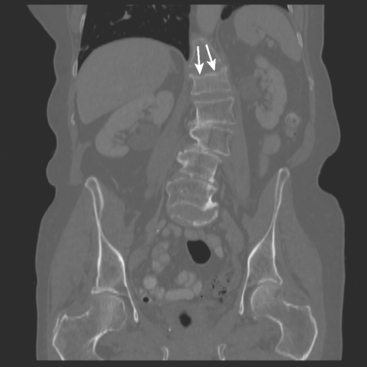
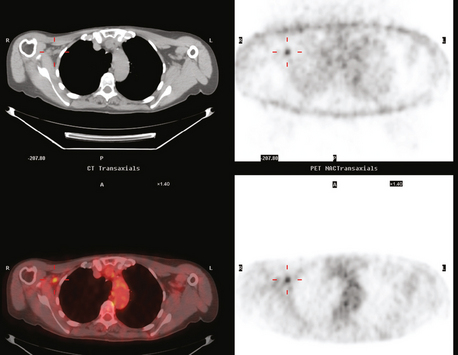
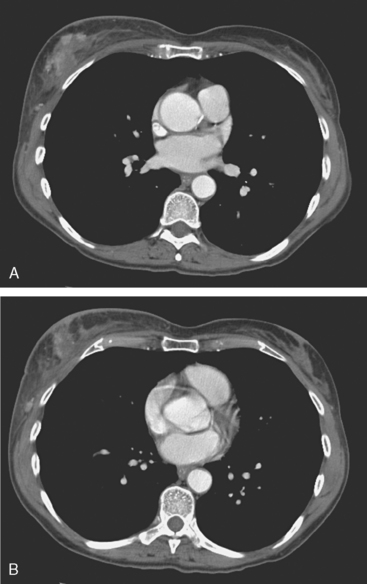
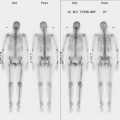
The most common sites of breast cancer metastasis are bone, lung, and liver, in that order.14 Evaluation of bone should begin with a careful history to elicit any symptoms or history of recent trauma. Serum alkaline phosphatase and calcium measurements may be helpful if positive and heighten suspicion of bony involvement. Imaging options, discussed in greater depth in Chapter 8, include bone scanning, CT, MRI, and PET. Bone scintigraphy, using a technetium-based agent (e.g., methylene diphosphonate), is exquisitely sensitive to changes in bone metabolism. Localization of these agents into bone is dependent on many factors, with the most important two being blood flow and osteoblastic activity. This results in a very predictable concentration in osteoblastic metastases (Figure 3). Positive findings on scintigraphy antedate findings on plain radiography by weeks to months. Drawbacks of bone scanning include suboptimal specificity, reduced sensitivity in osteolytic metastases, and persistent positivity at sites where active tumor is no longer present. Additionally, the well-recognized flare phenomenon, resulting in apparent worsening on scintigraphy due to treatment response, may mislead the unaware clinician or imager. Specificity is arguably the most problematic feature of whole-body skeletal scintigraphy. Any process that results in an increase in bone remodeling will demonstrate increased uptake of radiopharmaceutical. For this reason and because of the implications of labeling a patient as having bony metastases, correlative imaging (or even tissue sampling) is required. Correlation can be accomplished with plain radiography, CT, or MRI.
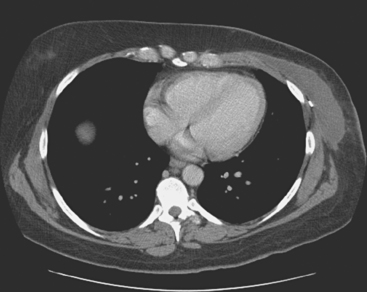
Plain radiography is of minimal value as a screening modality, requiring 30% to 50% loss of bone mineral for a metastasis to become visible.15 Plain radiographic correlation with a scintigraphic abnormality may be of benefit; however, the increased sensitivity and improved anatomic detail (including surrounding soft tissues) are factors favoring CT (Figure 4) or MRI. Although MRI has a higher rate of detection of skeletal metastases than scintigraphy in the spine, pelvis, limbs, sternum, scapulae, and clavicles,16 the logistics and cost of whole-body MRI give scintigraphy the edge as an initial screening examination.
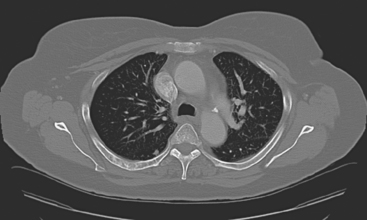
FIGURE 4 Chest CT image, displayed with bone windowing, from the same patient in Figure 3, shows abnormal, mottled, partially blastic bone mineral texture involving a long segment of a right posterior rib, corresponding to the bone scan.
PET and, more recently, PET/CT have been used to evaluate the entire body for metastases, including bone. An emerging consensus is that PET and scintigraphy have a similar sensitivity for detection of metastases, whereas PET shows a definite in-crease in specificity.17–20 There also appears to be a significantly higher sensitivity with fluorodeoxyglucose (FDG) PET for osteolytic metastases. Conversely, bone scintigraphy has shown superiority for demonstration of osteoblastic lesions. Currently, PET and bone scintigraphy are viewed as complementary imaging modalities for the detection of skeletal metastases.
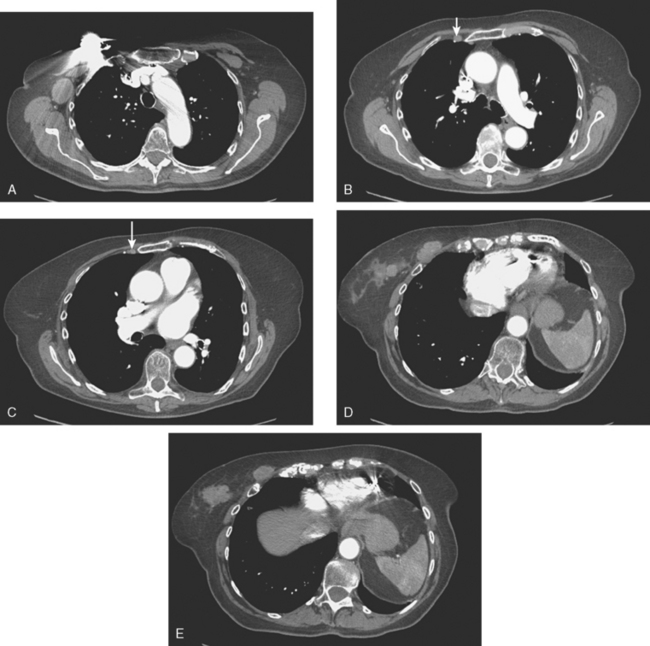
Lung metastases are relatively common in patients with metastatic disease and in those who die from breast cancer. However, lung metastases are very uncommon at initial diagnosis of breast cancer.21 Many centers still recommend a chest x-ray at initial screening (in part, because of the age of their breast cancer population); however, positive findings on chest x-ray generally necessitate CT correlation. CT is the modality of choice for chest evaluation. PET/CT offers advantages over CT alone in evaluating the mediastinum and as a whole-body survey.22
Evaluation of the liver is covered in depth in Chapter 9. In addition to CT, MRI, and PET, ultrasound may be appropriate in selected patients. The number of patients with hepatic metastases at initial presentation is extremely low. Screening, whether using liver enzymes or imaging, is nonspecific and of low diagnostic yield. For symptomatic patients and those with clinical evidence of liver involvement, CT and MRI are considered the imaging modalities of choice.23 Ultrasound may be useful to help characterize small lesions identified on CT.24 Finally, FDG PET is best viewed as complementary in the liver; it carries an excellent specificity and will occasionally find lesions not appreciated prospectively on CT or MRI, but it has limited sensitivity for small lesions (<1 cm) and can be difficult to interpret when there is heterogeneous FDG uptake, which can be exacerbated by attenuation correction (Table 2).
1 Haagensen C, Stout A. Carcinoma of the breast: Criteria of operability. Ann Surg. 1943;118:859-868.
2 Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual, 6th ed. New York: Springer Verlag, 2002.
3 Rieber A, Brambs HJ, Gabelmann A, et al. Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. Eur Radiol. 2002;12(7):1711-1719.
4 Partridge SC, Gibbs JE, Lu Y, et al. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol. 2002;179:1193-1199.
5 Rosen EL, Blackwell KL, Baker JA, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2003;181:1275-1282.
6 Londero V, Bazzocchi M, Del Frate C, et al. Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol. 2004;14(8):1371-1379.
7 Martincich L, Montemurro F, De Rosa G, et al. Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat. 2004;83(1):67-76.
8 Yeh E, Slanetz P, Kopans DB, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184(3):868-877.
9 Bassa P, Kim EE, Inoue T, et al. Evaluation of preoperative chemotherapy using PET with fluorine-18-fluorodeoxyglucose in breast cancer. J Nucl Med. 1996;37(6):931-938.
10 Schelling M, Avril N, Nahrig J, et al. Positron emission tomography using [(18)F]-fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18:1689-1695.
11 Biersack HJ, Palmedo H. Locally advanced breast cancer: is PET useful for monitoring primary chemotherapy? J Nucl Med. 2003;44(11):1815-1817.
12 Rosen EL, Eubank WB, Mankoff DA. FDG PET, PET/CT, and breast cancer imaging. RadioGraphics. 2007;27:S215-S229.
13 Xing Y, Ding M, Ross M, et al. Meta-analysis of sentinel lymph node biopsy following preoperative chemotherapy in patients with operable breast cancer. ASCO Annual Meeting, 2004, New Orleans, LA abstract 561.
14 Huston TL, Osborne MP. Evaluating and staging the patient with breast cancer. In: Ross D, editor. Breast Cancer. 2nd ed. Philadelphia: Elsevier Churchill Livingstone; 2005:309-318.
15 Schirrmeister H. Detection of bone metastases in breast cancer by positron emission tomography. PET Clin. 2006;1(1):25-32.
16 Chom Y, Chan K, Lam W, et al. Comparison of whole body MRI and radioisotope bone scintigram for skeletal metastases detection. Chin Med J (Eng1). 1997;110(6):485-489.
17 Cook GJ, Houston S, Rubens R, et al. Detection of bone metastases in breast cancer by 18 FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998;16(10):375-379.
18 Ohta M, Tokuda Y, Suzuki Y, et al. Whole body PET for the evaluation of bony metastases in patients with breast cancer: comparison with 99 m Tc-MDP bone scintigraphy. Nucl Med Commun. 2001;22(8):875-879.
19 Yang SN, Liang JA, Lin FJ, et al. Comparing whole body 18F-2 deoxyglucose positron emission tomography and technetium-99 m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128(6):325-328.
20 Uematsu T, Yuen S, Yukisawa S, et al. Comparison of FDG PET and SPECT for detection of bone metastases in breast cancer. AJR Am J Roentgenol. 2005;184(4):1266-1273.
21 Ciatto S, Pacini P, Azzini V, et al. Preoperative staging of primary breast cancer: a multicentric study. Cancer. 1988;61(5):1038-1040.
22 Dose J, Bleckmann C, Bachmann S, et al. Comparison of fluorodeoxyglucose positron emission tomography and “conventional diagnostic procedures” for the detection of distant metastases in breast cancer patients. Nucl Med Commun. 2002;23(9):857-864.
23 Reinig JW, Dwyer AJ, Miller DL, et al. Liver metastasis detection: comparative sensitivities of MR imaging and CT scanning. Radiology. 1987;162:43-47.
24 Eberhardt SC, Choi PH, Bach AM, et al. Utility of sonography for small hepatic lesions found on computed tomography in patients with cancer. J Ultrasound Med. 2003;22(4):335-343.
CASE 1 LABC (large tumor size)
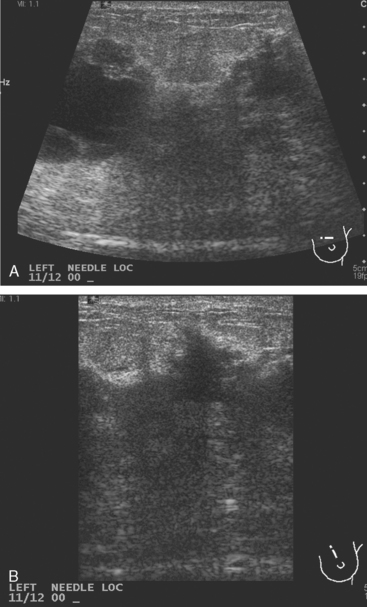
A 45-year-old woman was noted on routine physical examination by her physician to have an abnormal left breast examination. Although the patient had not noted any discrete masses, an area of induration and irregularity was palpated in the left upper breast, extending from upper inner to upper outer quadrant.
Breast imaging evaluation showed extremely dense breast parenchyma. New clustered microcalcifications were identified in the upper inner quadrant (UIQ). Sonography showed irregular, hypoechoic parenchymal echotexture in the left upper breast at 11 to 12 o’clock with cysts and shadowing (Figure 1), and the region of the microcalcifications was visualized as well. Ultrasound-guided biopsy confirmed infiltrating ductal carcinoma (IDC) with ductal carcinoma in situ (DCIS).
Breast MRI showed striking asymmetry between the two sides, with findings of a very large, diffusely infiltrating cancer on the left (Figures 2 and 3). The intensely enhancing process, centered at 12 o’clock but spanning 11 to 2 o’clock, showed architectural distortion, with additional extensive clumped enhancement diffusely involving the medial breast. By MRI, the abnormality measured at least 6 cm and involved at least two quadrants extensively, indicating that the patient was not a conservation candidate.
The mastectomy specimen showed an 8-cm IDC of the central breast extending into the upper inner and lower inner quadrants, with a high-grade DCIS component involving 50% of the lesion (extensive intraductal component). Multifocal satellite tumor nodules were noted within the large tumor. Extensive angiolymphatic involvement was seen, and four sentinel lymph nodes showed metastatic carcinoma (Figure 4). Completion axillary dissection showed no additional axillary disease, for a total of 4 of 20 lymph nodes positive for metastatic disease. The deep mastectomy margin was negative.
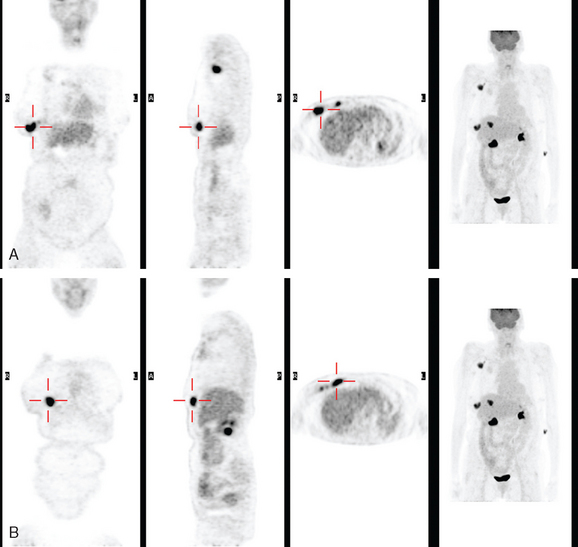
The patient underwent imaging staging postoperatively, with bone scan, CT, and positron emission tomography (PET) (Figures 5 and 6), which showed only postsurgical changes of the axilla and chest wall and no evidence of distant metastatic disease. Final stage was stage IIIA, with T3N1M0 disease, and the tumor was estrogen receptor and progesterone receptor positive.
TEACHING POINTS
Accurate and timely communication between specialties is a critical part of the care of the newly diagnosed breast cancer patient, as this case illustrates. Evaluations up to the point of the breast MRI had not shown clear evidence that the patient was not a lumpectomy candidate, and so the treatment planning was proceeding with this expectation. One examiner’s concern that there might be a discrepancy between the imaging findings and the clinical examination led to performance of breast MRI, which confirmed a very large, locally advanced tumor. Unfortunately, this was ordered at the last minute and without the knowledge of the surgeon. Fortunately, the radiologist charged with performing the needle localization on the day of surgery was able to rectify the situation, which was precipitously, but satisfactorily, resolved in favor of the patient undergoing mastectomy.
CASE 2 LABC with axillary and internal mammary involvement; staging with whole-body PET and PEM
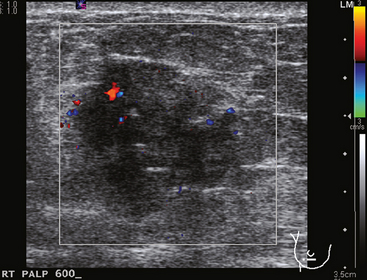
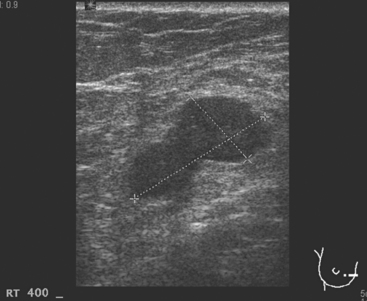
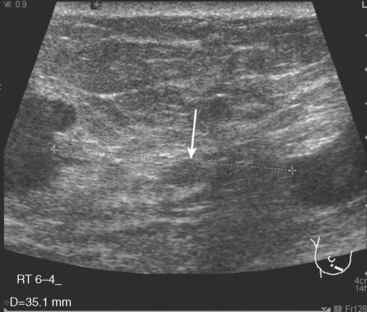
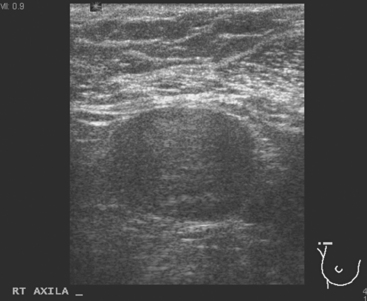
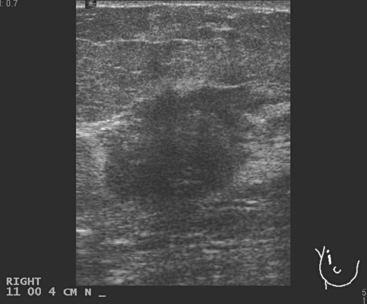
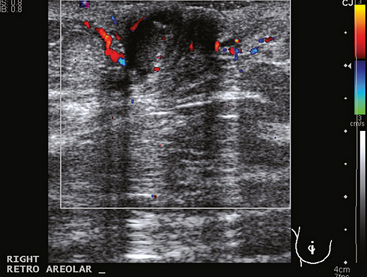
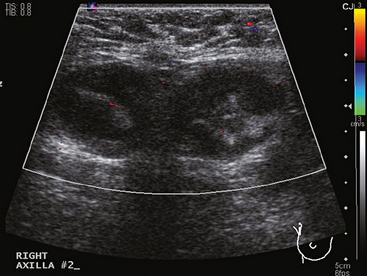
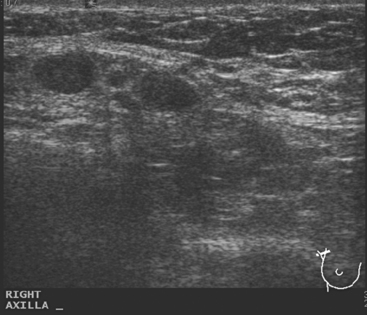
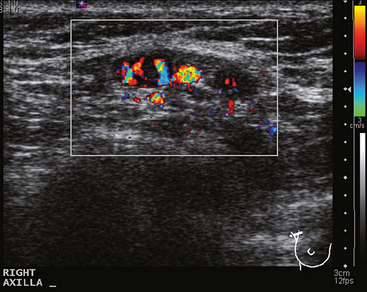
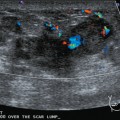
A 77-year-old woman was noted on routine physical examination by her primary care physician to have a palpable 3-cm mass on the right at 6 o’clock. Breast imaging evaluations confirmed this mass, which was best seen on ultrasound as a 3.2-cm mass with lobular and irregular margins (Figure 1). On ultrasound, a second suspicious mass, which was not seen mammographically, was noted medial to the dominant mass, at 4 o’clock (Figures 2 and 3). This was a bilobed, hypoechoic mass. A highly suspicious axillary lymph node was also found, measuring 2.3 cm, with complete effacement of the fatty hilus (Figure 4).
These three abnormalities were biopsied with ultrasound guidance. Both the 4- and 6-o’clock breast masses were poorly differentiated infiltrating ductal carcinomas (IDC), estrogen receptor and progesterone receptor negative, HER-2/neu negative, grade 8/9. The axillary lymph node fine-needle aspiration showed adenocarcinoma, consistent with metastatic breast carcinoma.
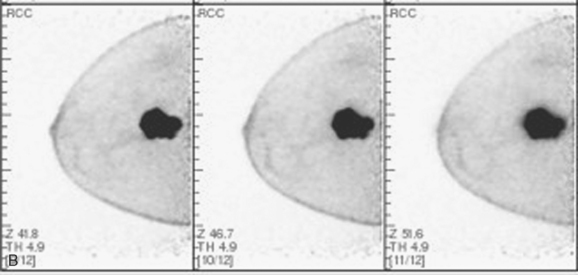
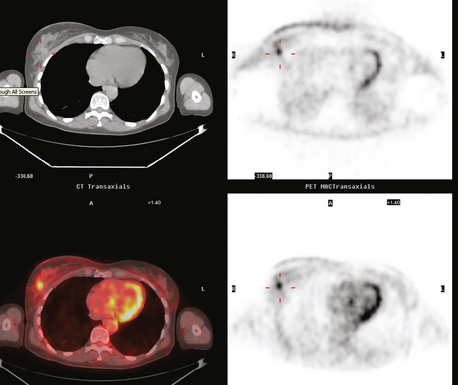
Bone scan suggested an L1 compression fracture as the etiology of back pain (Figure 5). A band of modest increased metabolic activity was seen at the superior end plate of L1 on PET, and CT showed superior end-plate invagination, confirming the bone scan impression of a compression fracture (Figure 6). Three right lower inner quadrant FDG-avid breast masses were seen on whole-body PET/CT, with a small additional FDG-avid nodule seen between the two known IDC masses at 4 and 6 o’clock. The known involved right axillary lymph node was intensely hypermetabolic and was accompanied by several smaller additional metabolically active axillary lymph nodes. Activity was also seen in two internal mammary lymph nodes, which on CT measured 8 mm (Figures 7 and 8).
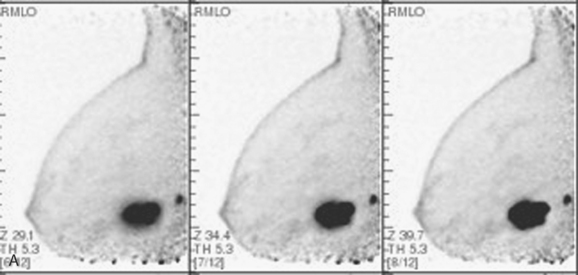
A PEM study was also obtained in this patient after completion of PET/CT, using the same FDG dose. The proven multifocality of her tumor would ordinarily have been an indication for breast MRI, but the patient could not readily undergo breast MRI because of her pacemaker. The dominant mass on the right at 6 o’clock was seen with exquisite detail on PEM, with intense heterogeneous uptake of FDG (Figure 9). The 4-o’clock mass was not seen on either view. This result was anticipated because of its far posterior position on the chest wall on CT. The small, intermediately positioned satellite tumor mass was visualized on the mediolateral oblique (MLO) projection, which shows more posterior breast tissue. The PEM also permitted more thorough screening of the left breast.
CASE 3 LABC with nipple skin involvement
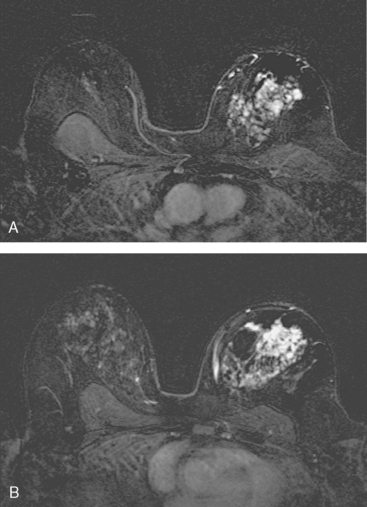
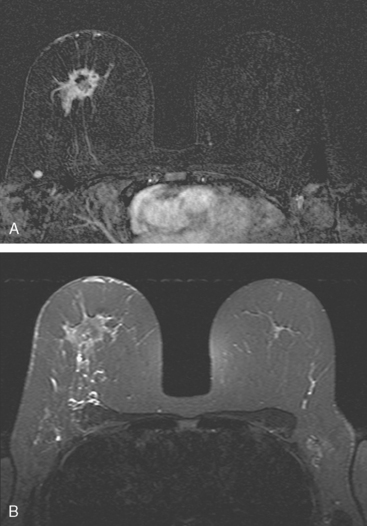
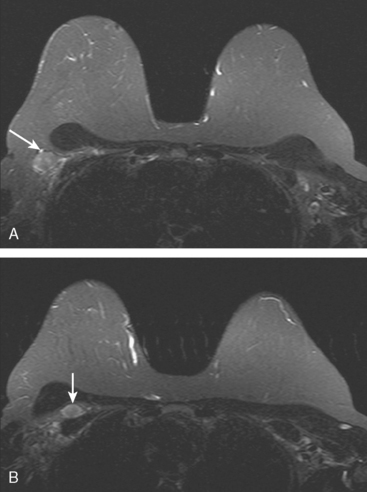
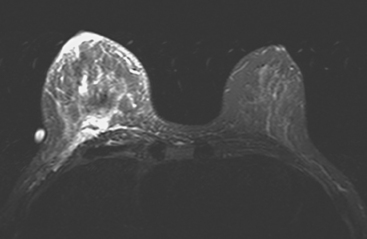
A 56-year-old woman noted new right nipple inversion 4 months before presenting for breast imaging evaluation for a newly developed right palpable axillary lump. Mammography and ultrasound showed a dominant 12-o’clock mass with nipple retraction and localized skin thickening, as well as axillary lymphadenopathy (Figures 1, 2, and 3). Ultrasound-guided biopsy of the dominant mass confirmed invasive ductal carcinoma (IDC), and ultrasound-guided axillary node fine-needle aspiration confirmed metastatic disease. Periareolar dusky erythema was noted, and skin punch biopsy was performed, which was negative. Local staging was completed with breast MRI (Figures 4, 5, 6, 7, and 8).
Systemic staging with positron emission tomography (PET)/CT showed fluorodeoxyglucose (FDG) avidity of the known right breast cancer and axillary lymphadenopathy, but no additional disease. Neoadjuvant chemotherapy was given, consisting of four cycles each of doxorubicin (Adriamycin) and cyclophosphamide (Cytoxan) (AC) and paclitaxel (Taxol). The nipple inversion resolved, and there was marked clinical regression of the dominant mass. Modified radical mastectomy and axillary dissection were performed. A 4.5-cm residual lesion of admixed IDC and normal breast tissue remained. Margins were negative, and 5 of 13 lymph nodes showed metastatic tumor. Chest wall, supraclavicular, and posterior axillary boost radiation therapy were given, and the patient was placed on anastrozole (Arimidex).
CASE 4 Natural history of untreated inflammatory breast cancer
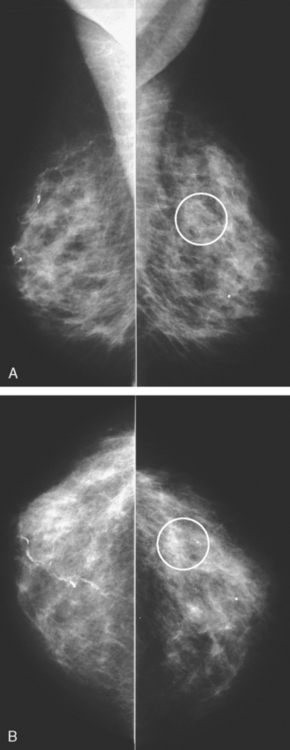
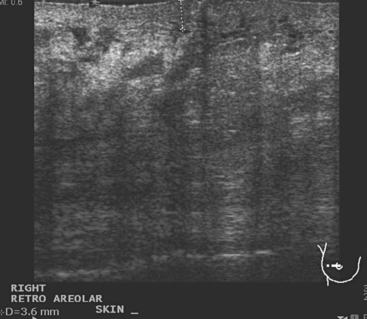
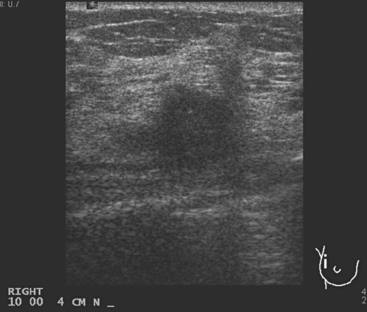
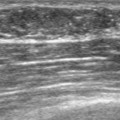
A 57-year-old woman noted her right breast to be enlarged and heavier feeling. Mammography showed concerning right upper outer quadrant (UOQ) microcalcifications (Figures 1 and 2). Right breast sonogram showed periareolar skin thickening (Figure 3) and an UOQ 1.5-cm shadowing hypoechoic mass with irregular margins (Figure 4), as well as hypoechoic, rounded, axillary lymph nodes (Figures 5 and 6). Ultrasound-guided core needle biopsy of the UOQ mass confirmed infiltrating ductal carcinoma (IDC), estrogen receptor and progesterone receptor negative, HER-2/neu negative. Ultrasound-guided fine-needle aspiration (FNA) of an axillary lymph node confirmed metastatic carcinoma, consistent with breast primary origin. Two skin punch biopsies were performed because of clinical suspicion of inflammatory carcinoma: both were negative. Skin biopsies were subsequently repeated because of persistent high suspicion of inflammatory cancer, and confirmed intralymphatic carcinoma.
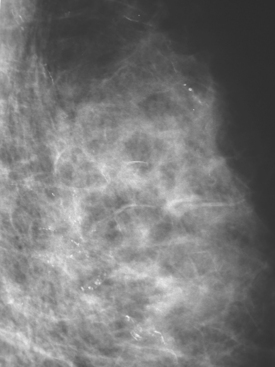
FIGURE 2 Lateral magnification view of the right upper breast shows concerning new microcalcifications.
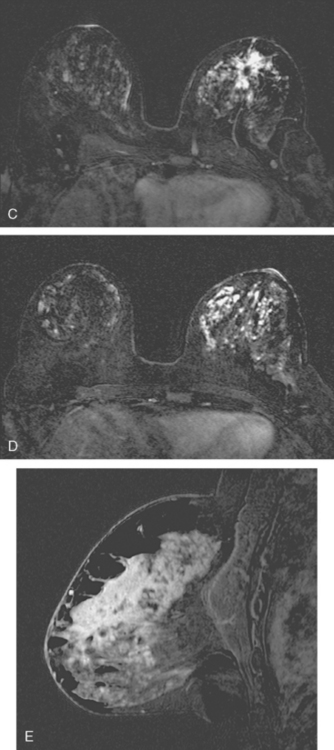
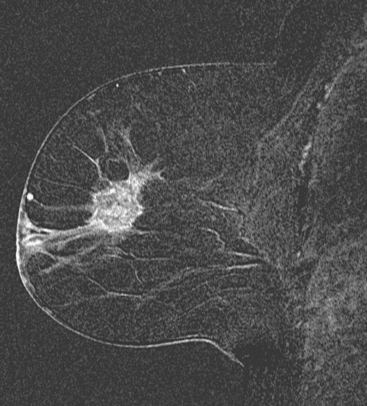
Breast MRI showed multiple intensely enhancing right breast masses, as well as enhancement of the skin (Figures 7, 8, 9, 10, and 11). Systemic staging consisted of positron emission tomography (PET)/CT and enhanced body CT scans. Hypermetabolism was identified in the right breast and axillary lymph nodes, but no evidence of distant metastatic disease was found (Figures 12, 13, 14, 15).
The patient initially declined all conventional therapies and opted against medical advice for a trial of an alternative soy product. After 3 months, she underwent repeat breast MRI and PET/CT to assess her response. The breast MRI showed growth of the multiple breast masses to near confluency (Figures 16 and 17). PET/CT showed progression in the breast and axilla, but no distant metastases. Four cycles of doxorubicin (Adriamycin) and cyclophosphamide (Cytoxan) (AC) neoadjuvant chemotherapy were given, after which the patient underwent a right modified radical mastectomy and axillary lymph node dissection. The mastectomy specimen showed a 5.5-cm IDC with high-grade comedo DCIS and dermal intralymphatic carcinoma. Angiolymphatic invasion was extensive, both peritumoral and distant. The margins showed intralymphatic carcinoma at skin margins. Fourteen out of 14 lymph nodes showed tumor, with extranodal soft tissue extension and involvement of perinodal lymphatic spaces.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree