CHAPTER 114 Lower Extremity Operations and Interventions
Vascular surgical procedures have traditionally been the “gold standard” against which newer technologies and their long-term results have been measured. Whereas open vascular surgical procedures have continued to undergo refinements and expansions of clinical indications, endovascular or catheter-based interventions have had an almost explosive growth of new technology and broadened indications and have increasingly gained acceptance as the primary treatment in a variety of applications. There are now long-term data for many of these endovascular procedures that compare favorably with the traditional open surgical operations.1–6 Some of the newer technologies continue to evolve and are likely to have expanding indications. The decision process for selection of open versus endovascular treatment, as well as which endovascular option, involves consideration of the specific disease entity, the medical condition and age of the patient, the anatomic constraints, and the durability of the procedure in question.
REVASCULARIZATION PROCEDURE: VASCULAR BYPASS SURGERY
Description and Special Anatomic Considerations
Vascular bypass surgery involves placement of a conduit to serve as an alternative vascular pathway to a diseased or obstructed arterial bed. Vascular surgical conduits are anatomically classified on the basis of the locations of the proximal and distal anastomoses. The most common infrainguinal bypass is the femoropopliteal bypass, between the common femoral and the popliteal arteries (Fig. 114-1A,B). The distal anastomosis may be to either the above-knee or the below-knee segment of the popliteal artery (Fig. 114-1C). Conduits are further defined according to the material from which they are constructed. They may be native, such as an autogenous vein or artery, or they may be prosthetic, such as expanded polytetrafluoroethylene (ePTFE) or Dacron. The native greater saphenous vein is preferred for bypass surgery in the lower extremity because it performs better than any other conduit choice. However, it may not always be an available option because donor veins may be diseased or may have been previously harvested for other vascular procedures, such as coronary artery bypass surgery. Other autogenous veins used as vascular conduits include the short saphenous vein, the femoral vein (also known as the superficial femoral vein) within the thigh, and the basilic and cephalic veins of the upper extremity.
The lack of a completely satisfactory prosthetic substitute for the greater saphenous vein has led to the use of other biologic conduits, such as human umbilical vein, arterial or venous homografts, and xenografts. There are certain inherent problems, such as aneurysmal degeneration (Fig. 114-2), and long-term patency issues that are unique to these biografts, and results remain mixed compared with prosthetic grafts.7
Extra-anatomic bypass refers to grafts that are constructed in anatomic locations that are significantly different from the normal location of the diseased arteries that are being bypassed. Typical examples are the cross-femoral (femorofemoral) and axillofemoral bypass grafts (Figs. 114-3 to 114-5). These were originally designed for patients too ill to undergo direct aortofemoral bypass or to replace grafts that were infected; these are still the primary indications. In addition, they now often serve as adjuncts to endovascular repair of abdominal aortic aneurysms (EVAR), particularly in the category of aorto–uni-iliac EVAR. These grafts are usually constructed of prosthetic material and generally have somewhat lower long-term patency than more traditional vascular bypass grafts, such as the aortobifemoral bypass graft. The obturator bypass (Fig. 114-6) was developed to replace femoropopliteal bypass surgery in patients with groin infections involving the native arteries or previously placed grafts and in patients with other complicating circumstances in the groin, such as trauma or previous radiation treatment. This bypass can be constructed with prosthetics or with autogenous vein.8
Indications
Patients with peripheral arterial disease may be classified by both a clinical description of the symptoms and objective testing criteria by the Rutherford categories of chronic limb ischemia (Table 114-1). These aid in prognosis and treatment planning.
Outcomes and Complications
Complications and lesions that threaten vascular conduit longevity include infection, development of anastomotic stenoses (Fig. 114-7) or pseudoaneurysms, progression of disease distal to the graft resulting in inadequate outflow, failure to incise all valves or to ligate all venous side branches within an in situ bypass graft, intimal hyperplasia, poor conduit quality, and degeneration of the graft. Such complications will require open surgical or endovascular correction.
The outcomes of various infrainguinal bypass procedures with use of currently available vascular conduits are summarized in Tables 114-2 to 114-4. With regard to extra-anatomic bypass grafts, the long-term patency rates are typically lower than for the anatomically positioned grafts. The obturator bypass graft for infrainguinal occlusive disease has reported patency rates of 73% and 57% at 1 and 5 years, respectively, which are somewhat lower than with conventional femoropopliteal bypass.8
REVASCULARIZATION PROCEDURE: ENDOVASCULAR TREATMENT OF STENOSIS AND OCCLUSIONS
Description and Special Anatomic Considerations
The most frequently employed endovascular treatment options are angioplasty and intravascular stent placement. Angioplasty may be used as a stand-alone primary therapy (Figs. 114-8 and 114-9) or may be combined with stent placement. Stents provide an intravascular scaffold for the vessel lumen and are available in a variety of materials, configurations, and delivery systems. Stents may be constructed from stainless steel, platinum, Elgiloy, and nitinol and may be combined with ePTFE or Dacron to produce a covered stent endoprosthesis or “stent graft.” Noncovered stents may be constructed with “open” or “closed cell” designs, which influence stent flexibility, conformability, radial strength, fracture resistance, and restenosis rates. In addition, there are balloon-mounted and self-expanding stents available in both the noncovered and covered groups (Figs. 114-10 and 114-11). Balloon-mounted stents are typically sized to correspond to the desired diameter of the vessel lumen, whereas self-expanding stents are usually oversized and may require secondary angioplasty to achieve a satisfactory diameter.
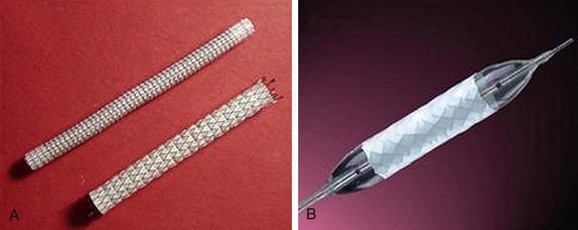
 FIGURE 114-11 Covered stents (stent grafts or vascular endoprostheses) are available as either self-expanding (A) or balloon mounted (B).
FIGURE 114-11 Covered stents (stent grafts or vascular endoprostheses) are available as either self-expanding (A) or balloon mounted (B).
Atherectomy involves a catheter-based atherosclerotic plaque excision system consisting of two components: a low-profile monorail excision catheter and a palm-sized power drive unit. A tiny rotating blade housed near the catheter tip is exposed when activated, removing and capturing thin shavings of plaque from the arterial wall into a collection chamber. Atherectomy thus permits “debulking” of atheroma from the lesion and may be used as a primary therapy or in combination with other endovascular treatment options. Catheters vary in diameter and tip length to accommodate various lesions (Figs. 114-12 and 114-13).
Cryoplasty is an angioplasty-based technology that uses liquid nitrous oxide as the balloon inflation medium, which lowers the balloon surface temperature to −10° C. Theoretically, cryoplasty causes an altered plaque response in which, as a result of freezing, microfractures form and weaken the plaque, contributing to a more uniform vessel dilation and less injury to the media. There may also be less elastic recoil and an induction of cellular apoptosis through freezing (Fig. 114-14).9,10
Another angioplasty-based technology is cutting balloon angioplasty, in which multiple small atherotomes (microsurgical blades) are fixed longitudinally on the outer surface of a noncompliant balloon. These expand radially during balloon inflation, delivering longitudinal incisions into the plaque and the vessel. Theoretically, there should be advantages to cutting balloon angioplasty through reduction of vascular injury by scoring of the vessel and the plaque longitudinally rather than by causing an uncontrolled disruption of the atherosclerotic plaque. However, in a randomized trial of 1385 coronary lesions, there was no significant difference between cutting and standard angioplasty at 6-month follow-up in angiographic and clinical results. The primary endpoint of angiographic restenosis at 6 months was 31.4% in the cutting balloon angioplasty group versus 30.4% in the standard group. This trial showed that cutting balloon angioplasty is equivalent in safety and efficacy endpoints to standard angioplasty, but it did not prove superiority for the general pool of percutaneous coronary intervention patients.11
Indications
The TransAtlantic Inter-Society Consensus Working Group (TASC) developed another classification system in 200012 and addressed both aortoiliac and infrainguinal occlusive disease, with the latter guidelines limited to femoropopliteal disease. These guidelines were updated in 200713 and reflect the expanded role of endovascular therapy as a primary option in the treatment of vascular occlusive disease (Table 114-5).
TABLE 114-5 TransAtlantic Inter-Society Consensus (TASC II) Recommendations (Femoropopliteal Arterial Disease)
| Lesion Category | Lesion Characteristics | Treatment Recommendation |
|---|---|---|
| Type A | Endovascular treatment | |
| Type B | Single or multiple lesions in the absence of continuous tibial vessels to improve inflow for a distal bypass |
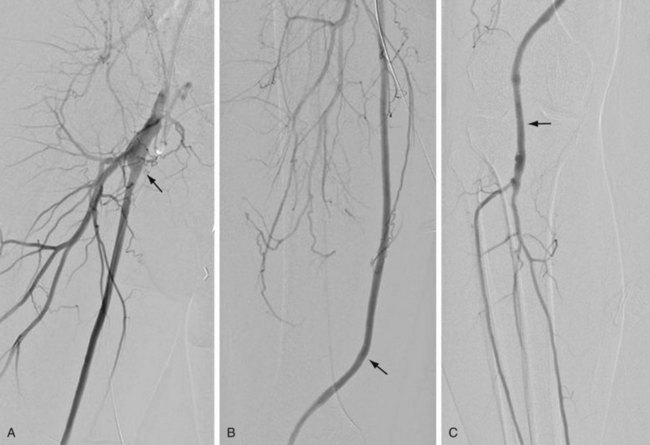
 FIGURE 114-1
FIGURE 114-1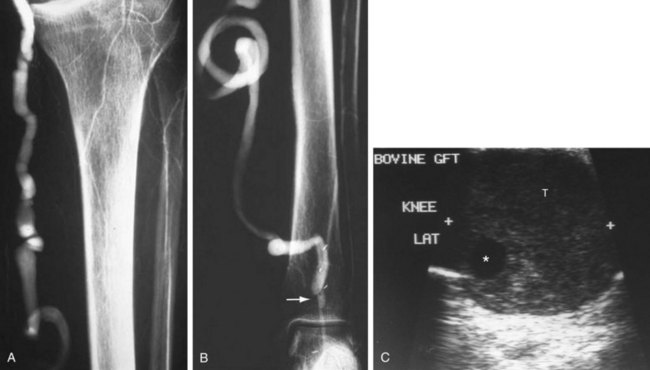
 FIGURE 114-2
FIGURE 114-2
 FIGURE 114-3
FIGURE 114-3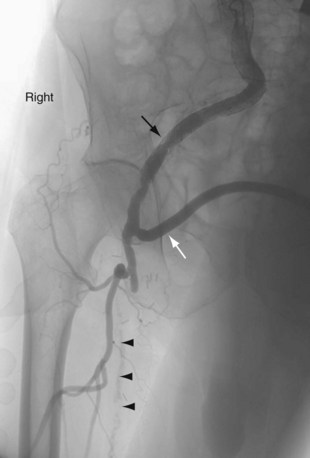
 FIGURE 114-4
FIGURE 114-4
 FIGURE 114-5
FIGURE 114-5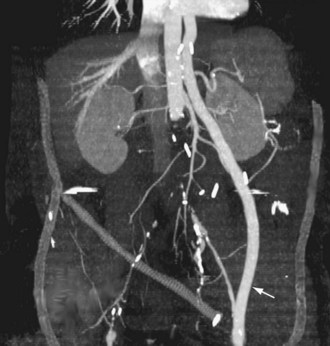
 FIGURE 114-6
FIGURE 114-6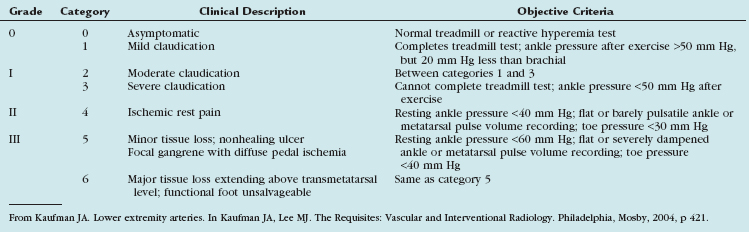
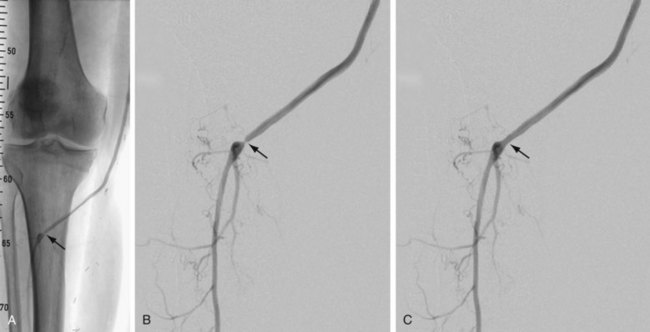
 FIGURE 114-7
FIGURE 114-7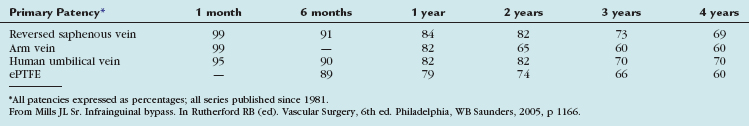
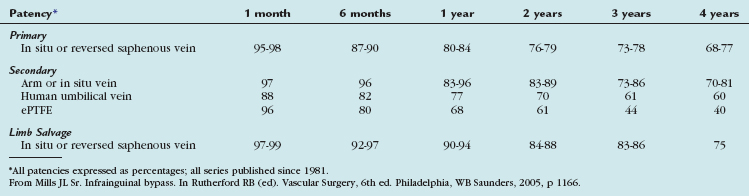
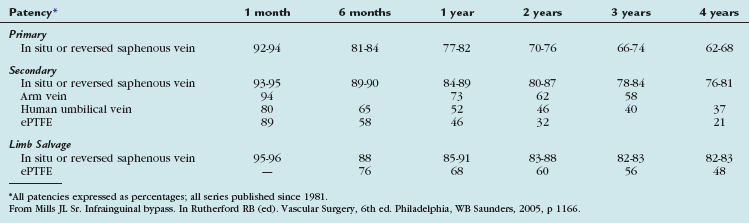

 FIGURE 114-8
FIGURE 114-8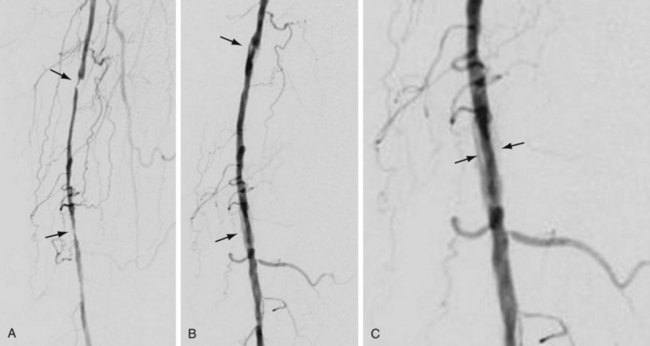
 FIGURE 114-9
FIGURE 114-9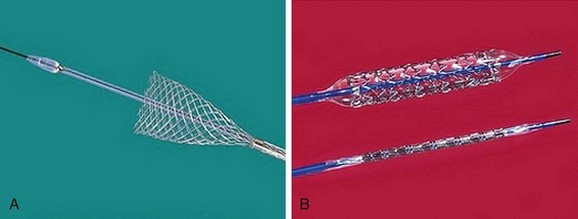
 FIGURE 114-10
FIGURE 114-10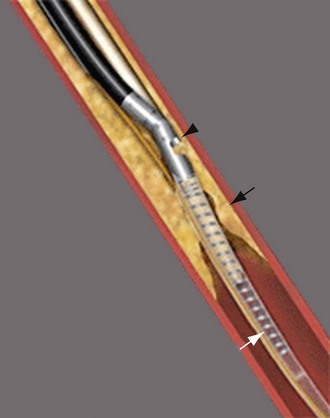
 FIGURE 114-12
FIGURE 114-12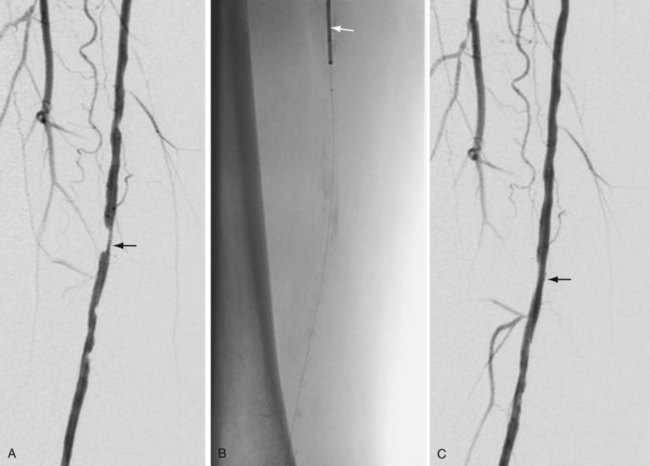
 FIGURE 114-13
FIGURE 114-13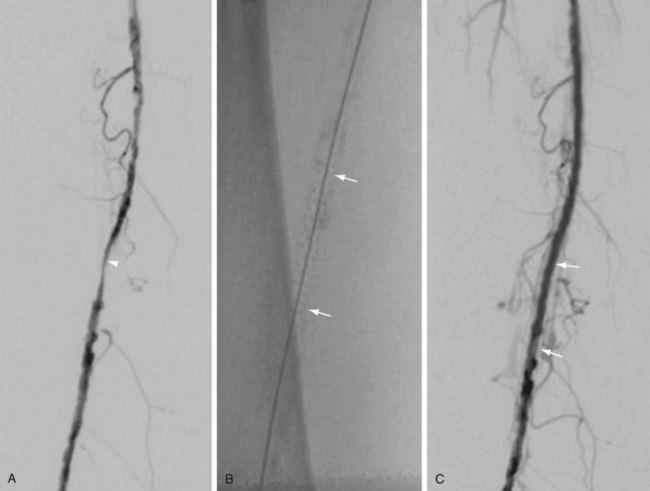
 FIGURE 114-14
FIGURE 114-14


