Repeat physical examination is warranted after a bout of provocative physical activity. One may find evidence of paresthesias, giving an indication as to which compartment is involved. Fascial herniations may be visualized. On palpation, the affected compartment will have increased tightness. In severe cases, muscle weakness and atrophy may be evident (George and Hutchinson 2012). In exceptional cases, CECS may progress into acute compartment syndrome if intensive sports participation is not stopped. Patients should be advised of this rare complication.
31.4.3 Diagnostic Investigation
Radiographs are unremarkable in patients with CECS. Compartment pressure testing is the gold standard for diagnosis. Post-exertional measures are required (Aweid et al. 2012). Pre-exertional measures may provide useful additional information (Paik et al. 2013). All four compartments should be routinely measured to prevent risk of recurrence and surgical failure. Many different methods have been described to measure the compartment pressure including slit catheter (Rorabeck et al. 1981), needle manometer (Whitesides et al. 1975), wick catheter (Mubarak et al. 1976), microcapillary infusion (Styf and Korner 1986), and microtip pressure method (McDermott et al. 1982). In a laboratory model, the arterial line manometer and Stryker devices (Fig. 31.2) are the most accurate (Boody and Wongworawat 2005). However, this has yet to be confirmed in vivo.
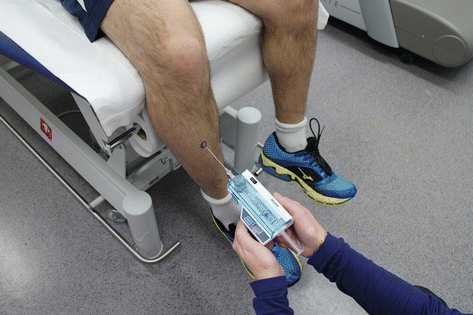

Fig. 31.2
Stryker intracompartmental pressure monitor
The criteria by Pedowitz are generally used in the diagnosis of CECS (Pedowitz et al. 1990). One of the following criteria must be present in addition to proper history and physical examination:
Resting pressure ≥15 mmHg
1 min postexercise pressure ≥30 mmHg
5 min postexercise pressure ≥20 mmHg
Whitesides proposed an alternate criterion, with a pressure increase within 20 mmHg of the diastolic blood pressure considered diagnostic (Whitesides and Heckman 1996).
Table 31.1 lists several other reported diagnostic criteria for CECS. It is important to note that intracompartmental pressure measurements are influenced by ankle and knee position and muscular tension (Gershuni et al. 1984). Clinicians are recommended to use a protocol with standardized catheter depth, exercise type, intensity and duration, footwear, and equipment. It may be wise to raise diagnostic thresholds to improve test specificity at the expense of sensitivity (Roberts and Franklyn-Miller 2012). Simultaneous intramuscular pressure and surface electromyography (EMG) measurement may prevent false diagnosis of CECS. The EMG detects remaining muscle contractions that elevate intracompartmental pressure (Zhang et al. 2011).
Table 31.1
Diagnostic criteria for chronic exertional compartment syndrome
Criteria | Pre-exercise | 1 min postexercise | 5 min postexercise |
|---|---|---|---|
Pedowitz | ≥15 | ≥30 | ≥20 |
Veith | >12 | >30 | >20 |
Hutchinson and Ireland | >10 | – | >25 |
Verleisdonk | – | ≥35 | – |
These are all invasive approaches, and recently interest has been drawn toward noninvasive pressure monitoring with the use of near-infrared spectroscopy and MRI. Near-infrared spectroscopy shows the deoxygenation of muscles during exercise and delayed reoxygenation postexercise in patients with CECS (Breit et al. 1997; Mohler et al. 1997; Zhang et al. 2001; van den Brand et al. 2004, Zhang et al 2012). MRI shows increased signal intensity in the involved compartment on T2-weighted sequence during exercise. If the compartment fails to return to baseline appearance within 25 min after exercise, it is considered diagnostic (Amendola et al. 1990; Verleisdonk et al. 2001). Other findings that could be seen on MRI are compartment bulging, effacement of fascial planes, convex deep fascial margins, and muscle herniation through fascial defects (Bresler et al. 2012). The usefulness of MRI for diagnosis has been called into question by Andreisek who found no difference in MRIs of healthy versus confirmed CECS patients at 3, 6, 9, 12, and 15 min postexercise (Andreisek et al. 2009).
31.4.4 Treatment
Nonsurgical management of CECS is frequently ineffective. Micheli et al. assessed multiple modalities including ice, rest, electrostimulation, and stretching and found that only prolonged rest and activity cessation were effective (Micheli et al. 1999). Recently, promising results have been reported with changing running technique in patients with CECS of the anterior compartment (Diebal et al. 2012). Anterior compartment pressures are significantly influenced by landing style (Kirby and McDermott 1983).
The use of compression sleeves (Fig. 31.3) is counterproductive, as they are not tolerated by patients with affirmed CECS. The sleeves increase already high intramuscular pressures and reduce the time of onset of complaints during exercise (Zimmermann 2013a).

Fig. 31.3
Sport compression sleeves
Commonly, a fasciotomy of the involved compartment is required, which can be performed through an open subcutaneous manner or an endoscopic approach (Ota et al. 1999; Leversedge et al. 2002; Lohrer and Nauck 2007; Wittstein et al. 2010). Consistent results are usually obtained after release of the anterior and lateral compartment (80 %) but not after release of the superficial and deep posterior compartment (50 %) (Howard et al. 2000; van Zoest et al. 2008). Fasciotomy is not without its short-term and long-term complications, which include hematoma formation, infection, scarring, venous thromboembolism, nerve injury, inadequate release, unrecognized nerve impingement (George and Hutchinson 2012), and venous insufficiency. Surgery for CECS reduces pain, but patients should be counseled that they may not return to their preinjury level of exercise or remain pain-free (Slimmon et al. 2002).
31.5 Popliteal Artery Entrapment Syndrome
Popliteal artery entrapment syndrome (PAES) is a rare entity but can be of devastating consequence if unrecognized and left untreated. The syndrome occurs most commonly in young active men (Stager and Clement 1999). The reported incidence is 0.2–3.5 % (Anil et al. 2011).
31.5.1 Etiology
PAES commonly results from abnormal anatomic relationship between the popliteal artery and the surrounding musculofascial envelope (Lambert and Wilkins 1999; Stager and Clement 1999). Four types of PAES have been described and classified according to Whelan-Rich (Love and Whelan 1965; Rich et al. 1979). Type 1 occurs when the popliteal artery has a course medial to the medial head of the gastrocnemius muscle. In type 2, the arterial course is normal but the medial head of the gastrocnemius arises from abnormal lateral position. In type 3, there is an abnormal slip of the muscle that arises from the gastrocnemius and compresses the popliteal artery. In type 4, there is an abnormal fibrous band or the popliteus muscle itself compresses the popliteal artery. Type 5, though not originally reported by Whelan, refers to involvement of the popliteal vein. Type 6 is when the anatomy is normal, but there is hypertrophy of the surrounding musculature compressing the artery (functional PAES) (Turnipseed 2009). In addition to the above, repeated trauma to the artery can result in damage to the arterial wall resulting in atherosclerosis or possible thrombus formation. Moreover, there is a possibility of formation of aneurysm distal to the constriction with resultant embolization of the thrombi.
Other potential vascular causes in athletes of lower limb pain include intimal hyperplasia, popliteal artery aneurysm, peripheral arterial dissections, and cystic adventitial disease (Pham et al. 2007).
31.5.2 Clinical Presentation
The typical history is of claudication pain in a young athlete, which may be associated with paresthesias. The pain is brought on by activity at a predetermined distance and usually relieved with rest. Rarely, there may be discoloration of the foot or toes. There is no pain at rest.
Physical examination may reveal abnormal pulses, fullness from an aneurysm, or a normal exam. In the latter case, it may be prudent to palpate the dorsalis pedis and/or tibial arterial pulse and compare pulses when the ankle is in neutral position, maximal dorsiflexion, and maximal plantar flexion. In suspected cases, investigation for an abnormal ankle brachial pressure index could be pursued (normal >0.9).
31.5.3 Diagnostic Imaging
Direct angiography is the gold standard to diagnose PAES. Though angiography can successfully diagnose the arterial constriction, it can shed little light on the exact cause of the stenosis. This is where MR angiography supersedes conventional angiography in that abnormal muscle origin or accessory muscle head could be identified. Moreover, MRI can provide real-time images with the patient performing active dorsiflexion and plantar flexion. Contrast-enhanced MR is helpful in dealing with turbulence and flow patterns in an aneurysm and can successfully delineate the correct lumen of the artery (Elias et al. 2003). It is recommended that MR examinations should be done in a bilateral setting as bilateral involvement is seen in 30–60 % of the patients (Anil et al. 2011). Computed tomography angiography is advantageous as both extremities could be scanned simultaneously with a single bolus and it is faster than MR (Anil et al. 2011).
31.5.4 Treatment
The treatment is almost always surgical with the exact operation guided by the abnormality present. It could either take the form of surgical excision of the offending structure, venous bypass, or an interpositional graft. Other alternatives include endoluminal revascularization (Meier et al. 2010).
31.6 Conclusion
Lower leg pain is a common occurrence in athletics. To determine the true etiology, the astute clinician must perform a detailed history and physical examination. Adjunctive testing can assist in confirming the diagnosis. MTSS is the most common cause of exertional lower leg pain. The patient will have diffuse posterior medial tibial pain, whereas the discomfort of a stress fracture is more focal. CECS causes reproducible lower leg pain with tightness and associated neuropathic findings in the affected compartment. Exertional compartment testing is required for diagnosis. In contrast, popliteal artery entrapment syndrome results in exertional claudication and can be confirmed on functional MR angiography. The treatment for MTSS and stress fracture is typically conservative, while that of CECS and PAES often requires surgical intervention.
References
Amendola A, Rorabeck CH, Vellett D, Vezina W, Rutt B, Nott L (1990) The use of magnetic resonance imaging in exertional compartment syndromes. Am J Sports Med 18(1):29–34PubMed
Anderson MW, Ugalde V, Batt M, Gacayan J (1997) Shin splints: MR appearance in a preliminary study. Radiology 204(1):177–180PubMed
Andreisek G, White LM, Sussman MS, Langer DL, Patel C, Su JW, Haider MA, Stainsby JA (2009) T2*-weighted and arterial spin labeling MRI of calf muscles in healthy volunteers and patients with chronic exertional compartment syndrome: preliminary experience. AJR Am J Roentgenol 193(4):W327–W333PubMed
Andrish JT, Bergfeld JA, Walheim J (1974) A prospective study on the management of shin splints. J Bone Joint Surg Am 56(8):1697–1700PubMed
Anil G, Tay KH, Howe TC, Tan BS (2011) Dynamic computed tomography angiography: role in the evaluation of popliteal artery entrapment syndrome. Cardiovasc Intervent Radiol 34(2):259–270PubMed
Aoki Y, Yasuda K, Tohyama H, Ito H, Minami A (2004) Magnetic resonance imaging in stress fractures and shin splints. Clin Orthop Relat Res April (421):260–267
Aweid O, Del Buono A, Malliaras P, Iqbal H, Morrissey D, Maffulli N, Padhiar N (2012) Systematic review and recommendations for intracompartmental pressure monitoring in diagnosing chronic exertional compartment syndrome of the leg. Clin J Sport Med 22(4):356–370PubMed
Banal F, Gandjbakhch F, Foltz V, Goldcher A, Etchepare F, Rozenberg S, Koeger AC, Bourgeois P, Fautrel B (2009) Sensitivity and specificity of ultrasonography in early diagnosis of metatarsal bone stress fractures: a pilot study of 37 patients. J Rheumatol 36(8):1715–1719PubMed
Barry NN, McGuire JL (1996) Acute injuries and specific problems in adult athletes. Rheum Dis Clin North Am 22(3):531–549PubMed
Batt ME, Ugalde V, Anderson MW, Shelton DK (1998) A prospective controlled study of diagnostic imaging for acute shin splints. Med Sci Sports Exerc 30(11):1564–1571PubMed
Beck BR (1998) Tibial stress injuries. An aetiological review for the purposes of guiding management. Sports Med 26(4):265–279PubMed
Beck BR, Osternig LR (1994) Medial tibial stress syndrome. The location of muscles in the leg in relation to symptoms. J Bone Joint Surg Am 76(7):1057–1061PubMed
Bennell KL, Brukner PD (1997) Epidemiology and site specificity of stress fractures. Clin Sports Med 16(2):179–196PubMed
Bennell K, Matheson G, Meeuwisse W, Brukner P (1999) Risk factors for stress fractures. Sports Med 28(2):91–122PubMed
Bennett JE, Reinking MF, Pluemer B, Pentel A, Seaton M, Killian C (2001) Factors contributing to the development of medial tibial stress syndrome in high school runners. J Orthop Sports Phys Ther 31(9):504–510PubMed
Boden BP, Osbahr DC (2000) High-risk stress fractures: evaluation and treatment. J Am Acad Orthop Surg 8(6):344–353PubMed
Boden BP, Osbahr DC, Jimenez C (2001) Low-risk stress fractures. Am J Sports Med 29(1):100–111PubMed
Boody AR, Wongworawat MD (2005) Accuracy in the measurement of compartment pressures: a comparison of three commonly used devices. J Bone Joint Surg Am 87(11):2415–2422PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree