Fig. 1
Biocontinuum of adverse early and late effects are shown of the lung (with permission from Rubin and Casarett 1968)
2 Anatomy and Histology
2.1 Gross Anatomy
The lungs are paired organs, each contained in its own pleural sac within the thoracic cavity. Both lungs are subdivided into anatomically distinct lobes. The right lung normally has three lobes separated by two fissures. The horizontal fissure separates the upper and middle lobes while the oblique fissure separates the lower lobe from the middle and upper lobes. The left lung normally has two lobes (upper and lower) divided by an oblique fissure. The lobes can be further subdivided into segments. The lingula (Latin-little tongue) is part of the upper lobe and is composed of a superior and inferior segments (Fig. 2).
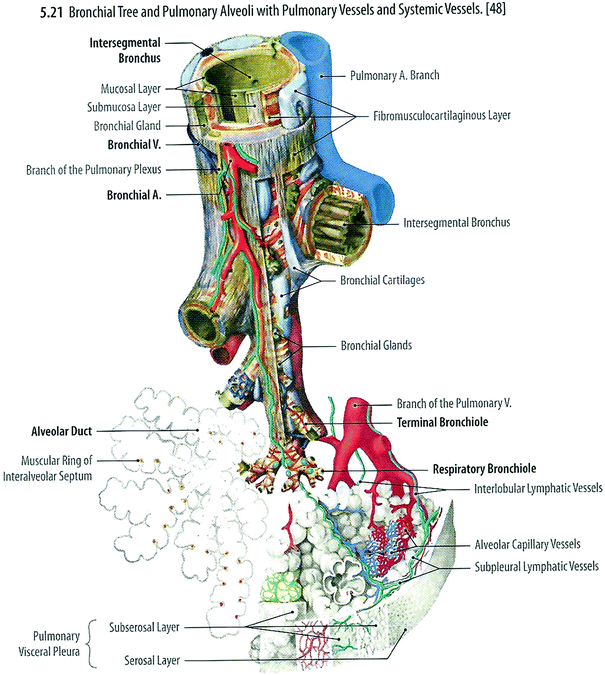

Fig. 2
Gross anatomy of the lung (with permission from Tillman)
The architecture of the lung can be considered as subunits arranged in both parallel and series. Centrally, the structure is “in series” as the large vessels and airways in the mediastinum and hilum support all of the distal vessels and airways to which they are connected. Fibrosis/stenosis of a central vessel or bronchus will render the downstream alveoli nonfunctional. Conversely, in the periphery, the smaller alveolar/capillary units function relatively independently (i.e., “in parallel”). Resection or injury to a region of lung will not compromise function of adjacent regions. The alveolar/capillary units appear far more sensitive to the effects of RT than the central conducting airways and vessels. Therefore, most RT-induced injury is referable to these “peripheral” structures and the lung is classically considered a “parallel” organ regarding RT-induced effects.
2.2 Histology and the Functional Subunit
The functional unit of the lung is the pulmonary lobule which consists of a terminal bronchiole and the lung parenchyma distal to it (i.e., alveolar/capillary unit) (Fig. 3a, b). The terminal bronchiole is the final conducting branch of the tracheobronchial tree not involved in gaseous exchange. Terminal bronchioles branch into transitional airways (respiratory bronchioles and alveolar ducts) which contribute to gaseous exchange. The transitional airways terminate in alveolar sacs which open into the alveoli.
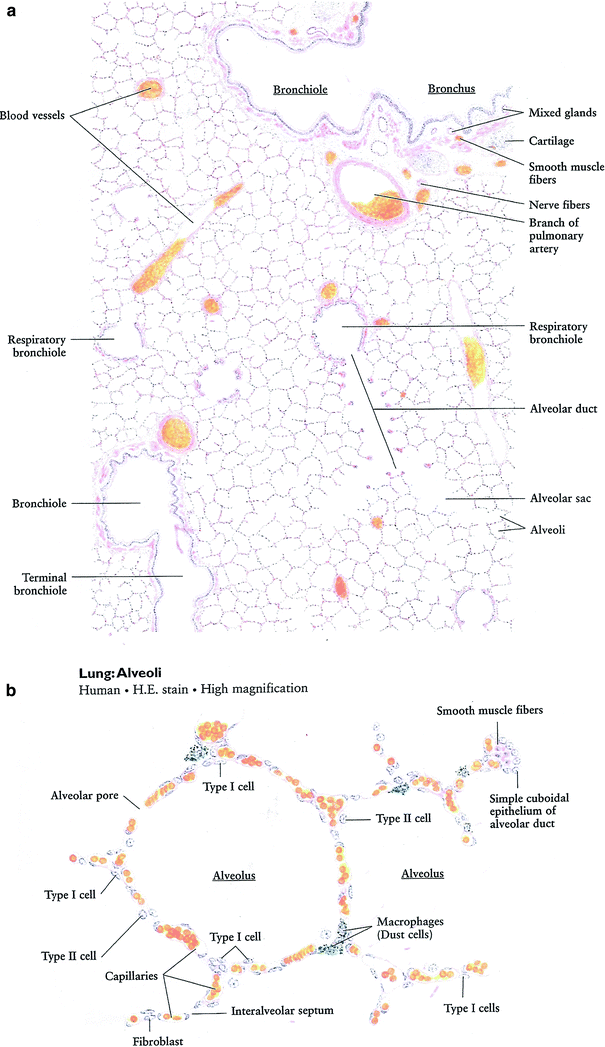

Fig. 3
Lung histology. a Low magnification, b high magnification (with permission from Zhang)
The principal function of the lungs is to facilitate gas exchange between alveoli and adjoining capillaries. Gas exchange is most efficient when the thickness of intervening tissues is minimal. Adjacent alveoli share a common wall, termed the interalveolar septum, which contains capillaries and a fine connective tissue framework. Within the interalveolar septum, the adjacent basal lamina of the alveolar epithelium and endothelium are often fused providing a thin barrier between the erythrocytes in the capillaries and the alveolar air space (~0.5 μm). The capillaries wrap around the alveoli creating a prodigious surface area for diffusion (50–100 m2).
Several cell types are present in the pulmonary lobule. Type 1 pneumocytes are flat epithelial cells with minimal cytoplasm and few organelles that form a complete, thin (0.2 μm), lining of the alveoli. Type II pneumocytes are as numerous as type I pneumocytes but only cover 2–5% of the alveolar surface. They are round cells with larger nuclei, and contain prominent lamellar bodies (cytosomes) which contain dipalmitoyl phosphatidylcholine (surfactant), which reduces surface tension within the alveoli. Type II pneumocytes can differentiate into type I pneumocytes in response to damage to the alveolar lining. Alveolar macrophages (dust cells) derive from circulating monocytes and phagocytose microorganisms and other particulate matter that is deposited in the alveoli.
3 Biology, Physiology, and Pathophysiology
3.1 Biology: Molecular Mechanisms of RT-Induced Lung Injury
Radiation injury of the lung is categorized as two distinct phases: acute inflammatory (pneumonitis) and late fibroproliferative. The progression of radiation injury is well established in both mice and humans. In humans, the pneumonitis phase peaks between 2–3 months after radiation exposure. This phase is characterized by a dense inflammatory cell infiltrate, exudation of proteinaceous material, and edema. The chronic fibroproliferative phase occurs months to years after exposure and is characterized by diffuse interstitial fibrosis, focal scarring, and impaired ventilation.
The lung is considered to be a late responding tissue, as the time between exposure and symptomatic injury can last months to years, presumably as a result of innate tissue kinetics. During the asymptomatic latency period, radiographic changes can be observed (Skoczylas et al. 2000; Brush et al. 2007; Fleckenstein et al. 2007). Although the period of time between exposure and symptomatic injury may appear to be quiet, there is a firestorm of events occurring at the molecular and cellular level that is just beginning to be better understood.
At the time of the initial irradiation, exposed cells rapidly spread the “danger signal” to neighboring cells as far away as 1 mm (Tsoutsou and Koukourakis 2006; Pusey 1905). This results in an orchestrated response of tissue-specific cells, the vascular endothelium, and inflammatory mediators in order to resolve the injury (Skoczylas et al. 2000; Fleckenstein et al. 2007; Tsoutsou and Koukourakis 2006; Bernier et al. 2004).
The intercellular conversation is initiated at the moment of irradiation when injury to the cell membrane, DNA, cytoplasmic bodies, and other cell components occurs, resulting in an immediate release of cytokine mRNA. The identification of a “perpetual cytokine cascade” (Fleckenstein et al. 2007) caused a major paradigm shift from the classical “target cell theory” (α/β) to one that posits a dynamic molecular crosstalk among numerous cells in normal tissue (the lung has more than 40 cell types (Evans and Leucutia 1925)) that propagates the injurious process (Fig. 4).
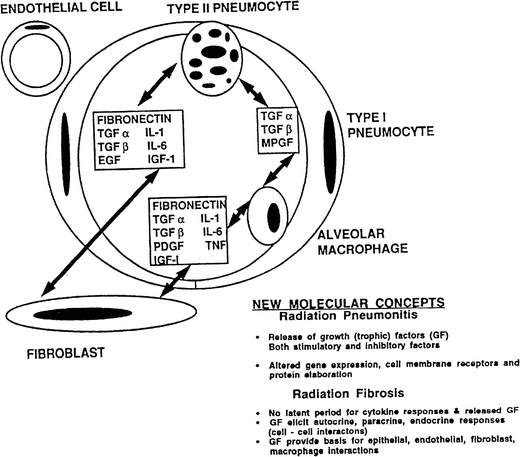

Fig. 4
Cytokine cascade associated with lung injury. Cell–cell interactions and control of gene expression by growth factors among alveolar cells is shown (with permission from McDonald et al. 1995, IJROBP LENT SOMA)
The cytokine and chemokine reaction observed by Rubin and colleagues clearly demonstrated the absence of a molecular and cellular latent period prior to observed histologic changes. These findings suggest that radiation initiates a downstream sequence of spatial and temporal events that eventually express themselves as clinical manifestations months later.
Coincident with the release of inflammatory cytokines is the development of oxidative stress. Free radical species can participate in a host of cellular signaling events. Indeed, many of the signaling pathways implicated in radiation injury, such as the TGF-β pathway, are sensitive to changes in cellular redox status (Tyler and Blackman 1922; Groover et al. 1922). The transient nature of ionizing radiation suggests that a mechanism of amplification of ROS/RNS occurs after radiation. Proposed mechanisms include activation of oxidant-generating enzymes, such as NADPH oxidases, mitochondrial leakage, and the respiratory burst from activated inflammatory mediators recruited to the site of injury. The role of NADPH oxidases is supported by the fact that TGF-β is activated by radiation and that in turn, TGF-β can activate the NADPH oxidase isoform, Nox4. Newer data coming to light recently have shown a critical role for Nox4 in TGF-β mediated apoptosis (Hines 1922) and downstream Smad signaling (Davis 1924).
In the lung, chronic oxidative stress has been observed using markers for DNA oxidation (Skoczylas et al. 2000) and lipid peroxidation (Groover et al. 1922; Fleckenstein et al. 2007). Oxidative stress can contribute to a number of changes within injured tissue including endothelial cell activation, increased vascular permeability and edema, lipid peroxidation, inflammation, and fibrosis (Skoczylas et al. 2000; Bernier et al. 2004; Johnston et al. 1996; Rubin et al. 1995). The end result is a cyclic progression of oxidative stress and activation of growth factors, transcription factors, and cytokines, all of which can contribute to lung injury.
It is hypothesized that the imbalance between oxidant-generating enzymes and antioxidant capacity is a primary cause of hypoperfusion following radiation (Skoczylas et al. 2000; Vujaskovic et al. 2001). Fleckenstein et al. demonstrated that tissue perfusion begins to decrease 3 days after radiation exposure, resolves for approximately 2 weeks, and then progressively decreases throughout the time of disease progression (Skoczylas et al. 2000). In parallel, the lung tissue becomes increasingly hypoxic (Skoczylas et al. 2000). The late decrease in tissue oxygenation is likely a result of fibrous obliteration of capillaries and decreased gas exchange due to increased alveolar wall thickness and edema/congestion within the alveolar space. The early drop in perfusion cannot be explained by these events and is more likely a result of vasoconstriction, although this is not confirmed. The activation of NADPH oxidase after radiation increases superoxide production, which may suppress vasodilation by oxidizing key enzymes within the NO signaling pathway or through rapid reaction with NO itself to form peroxynitrite. Both of these reactions can interfere with NO signaling leading to vasoconstriction, a transient loss in tissue perfusion, and tissue hypoxia. Tissue hypoxia, a fundamental feature underlying the development of lung injury, develops several weeks after lung irradiation and progresses with time, presumably as a result of injury to the pulmonary vascular and decreased perfusion (Skoczylas et al. 2000; Bernier et al. 2004). Changes in pulmonary perfusion have been observed within months following high doses of radiation in humans (Bentzen 2006; Mikkelsen and Wardman 2003) and within weeks in rodent models (Skoczylas et al. 2000; Rubin et al. 1995; Jackson et al. 2006). In studies by Fleckenstein et al. (Skoczylas et al. 2000), the transient decrease in perfusion was associated with increased edema and elevated expression of hypoxia-inducible factor-1 alpha (HIF-1α) and TGF-β. In that study, the vascular perfusion changes were noted before the development of histologically apparent and functional disease.
The same study showed that tissue hypoxia and perfusion changes coincide with the accumulation and activation of macrophages within the residual alveolar space and interstitium, as well as enhanced cytokine and growth factor production. This could, in part, contribute to the presence of cytokines in the irradiated tissue weeks to months after exposure (Skoczylas et al. 2000). Jackson et al. (Johnston et al. 1996) found that in vitro TGF-β production by macrophages could be induced by hypoxia (0.5% O2), prior to the onset of VEGF production. In that study, incubation with a superoxide dismutase mimetic led to decreased TGF-β and VEGF production (Johnston et al. 1996). Thus, it appears that ROS signaling plays a key role in macrophage-associated expression of the above cytokines under hypoxic conditions.
The pro-inflammatory/pro-fibrogenic cytokine transforming growth factor-beta (TGF-β) has been most extensively studied in regard to its role in the development of radiation-induced lung injury (Fleckenstein et al. 2007; Novakova-Jiresova et al. 2007; Kang et al. 2003; Feletou et al. 1995). TGF-β is secreted as an inactive polypeptide by a number of cells including macrophages, fibroblasts, and epithelial cells of the lung. The inactive precursor is sequestered in the extracellular matrix with its latency-associated peptide (LAP), which has been suggested to be the “sensory” portion of the protein (Marks et al. 1997; Theuws et al. 2000; Peterson et al. 1992; Dor et al. 2001; Li et al. 2001). The LAP is sensitive to free radicals, changes in cellular pH, and integrins, all of which can induce activation of TGF-β which readily binds the TGF-β type II receptor to stimulate the Smad second messenger signaling pathway (Bartholdi et al. 1997). Activation of TGF-β and subsequent translocation of Smad2/3 protein into the nucleus of the responding cell leads to transcription of genes involved in fibroproliferation and tissue repair (Li et al. 2007; van Hinsbergh et al. 2001). An increase in TGF-β protein can be observed within 24 hours following radiation exposure (Skoczylas et al. 2000; Dor et al. 2001; Anscher et al. 1998, 2006). Epperly et al. (Rodemann et al. 1996) found a biphasic TGF-β surge associated with the early and delayed radiation response in mice after 20 Gy whole lung irradiation. The establishment of TGF-β as a mediator of radiation-induced lung injury has led to a number of studies evaluating circulating serum TGF-β levels as a potential biomarker for increased risk of lung injury as well as pre-clinical testing of inhibitors of TGF-β and/or its signaling pathway. These studies have used either neutralizing antibodies or adenoviral vectors to demonstrate significantly reduced histological and functional damage in rodent models of lung injury following radiation exposure. Reduced lung damage was also associated with decreased cytokine activity, fewer macrophages, and less oxidative stress (Kang et al. 2003; Barcellos-Hoff 1996; Rahimi and Leof 2007).
In summary, our knowledge of the mechanisms of radiation injury has vastly improved over the past 30 years. It is now established that chronic oxidative stress and continuous production of cytokines, particularly TGF-β, play a significant role in sustaining inflammation and fibroproliferation in irradiated lung during the progression of disease. Symptomatic and histologically relevant lung injury is preceded by cytokine production and communication, inflammatory cell infiltration, changes in blood flow and perfusion, and increased vascular permeability (Bernier et al. 2004; Roberts 1999; Barcellos-Hoff 1993; Barcellos-Hoff et al. 1994; Stone et al. 2003, Vujaskovic et al. 2000). Although great progress has been made in identifying the mechanisms underlying radiation-induced lung injury, more comprehensive studies are needed to link the events occurring at the molecular level to cellular and tissue pathology.
Despite these laboratory findings, the results in the clinic are less encouraging. To date, the observed associations between changes in a variety of biological markers and clinical pneumonitis have been inconsistent (Table 1).
Table 1
Proposed biological markers of lung injury
|
Marker
|
Author (n)
|
Increased when?
|
Predictive for RP?
|
|---|---|---|---|
|
Interleukins
|
|||
|
IL-1α
|
Before, during, after RT
|
Yes
|
|
|
IL-6
|
Before, during, after RT
|
Yes
|
|
|
IL-6
|
Post-RT
|
Weakly
|
|
|
IL-6
|
Before RT
|
No
|
|
|
IL-8
|
Before RTa
|
Yes
|
|
|
Growth factors
|
|||
|
TGF-β1
|
End of RT (relative to pre-RT)
|
Yes
|
|
|
TGF-β1
|
End of RT (relative to pre-RT)
|
Yes
|
|
|
TGF-β1
|
End of RT (relative to pre-RT)
|
Yes
|
|
|
TGF-β1
|
Mid-RT (relative to Pre-RT)
|
Weakly
|
|
|
TGF-β1
|
Before, during, after RT
|
No
|
|
|
TGF-β1
|
Any time
|
No
|
|
|
TGF-β1
|
Pre- RT
|
No
|
|
|
TGF-β1
|
Before RT
|
No
|
|
|
b FGF
|
Before, during, after RT
|
No
|
|
|
Others
|
|||
|
ACE
|
Pre- and Post-RT
|
Yes
|
|
|
TM
|
During RT
|
Yes
|
|
|
MCP-1
|
Before, during, after RT
|
No
|
|
|
E/L selectin
|
Before, during, after RT
|
No
|
|
3.2 Pathophysiology (Cellular Dynamics and the Radiation Response)
3.2.1 Animal Models
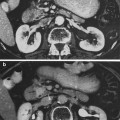
The ultrastructural changes in lung were documented by Penney et al. in a mouse model. These are documented for a wide range of single doses from 5 to 13 Gy over a period of months (1 h–60 weeks) (Table 2) (Penney 1087). Examples of corresponding histologic images are shown in Fig. 5a, b.
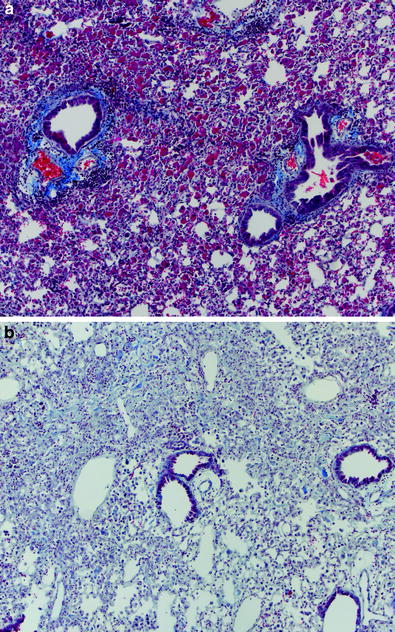
Table 2
Changes in the lung after radiation therapy (Adapted from Clinical Oncology 8th edition)
|
Dose (Gy)
|
|||||
|---|---|---|---|---|---|
|
Event
|
Time
|
0
|
5
|
9
|
13
|
|
Type II cell degranulation; surfactant release
|
1 h–7 days
|
–
|
–
|
–
|
+
|
|
Loss of basal laminar proteoglycans
|
1 h–7 days
|
–
|
±
|
+
|
++
|
|
Loss of alveolar macrophages
|
7 days
|
–
|
–
|
±
|
+
|
|
Surfactant recovery
|
4 weeks
|
–
|
–
|
–
|
+
|
|
Replacement of laminar proteoglycans
|
4–12 weeks
|
–
|
±
|
±
|
+
|
|
Radiation pneumonitis
|
12–30 weeks
|
–
|
–
|
–
|
+
|
|
Peak in soluble fibronectin
|
12–30 weeks
|
–
|
±
|
±
|
++
|
|
Fibrosis
|
30–60 weeks
|
–
|
±
|
+
|
+
|
|
Peak in insoluble fibronectin
|
30–60 weeks
|
–
|
+
|
+
|
++
|
|
Increased laminin
|
12–60 weeks
|
–
|
±
|
+
|
++
|

Fig. 5
a Radiation pneumonitis: pneumonitis in a C57L/J mouse 14 weeks after thoracic irradiation with a single dose of 10 Gy. Alveolar inflammation and perivascular lymphocytic cuffing can be seen. b Radiation fibrosis in a C57BL/6J mouse 24 weeks after thoracic irradiation with a single dose of 12.5 Gy. Both are Masson’s trichrome stain of paraffin embedded tissues
The Biocontinuum ‘roller coaster’ curve for radiation pneumonitis hypothesized by Rubin and Casarett was confirmed in an elegant series of studies in mice using breathing rates by Travis et al. (1980). The increased breathing rates at 16–36 weeks are associated with radiation pneumonitis and then becomes elevated again at 52 weeks associated with fibrosis. Utilizing a split dose exposure, the early pneumonitis phase was not expressed, however, the late occurrence of pulmonary fibrosis appeared between 6–12 months. This was confirmed in similar studies by Siemann et al. (1982) who utilized different fractionation schedules in mice and confirmed the appearance of a late fibrotic stage without an early pneumonitic phase. There observations support the concept of differing underlying mechanisms for pneumonitis and fibrosis.
The importance of host response is well-recognized clinically. A number of investigators have compared the variation in pulmonary response comparing a radioresistant strain (C3H) with a radiosensitive strain (C57BL) to radiation (Jackson et al. 2010; Williams et al. 2010). The basis for the variation could be explained by differences in the elicited cytokine cascade to similar doses. Utilizing knock out mice for 1 CAM expression compared to wild type mice, Hallahan demonstrated a lower incidence of lung injury due to blocking the 1 CAM-1 pathway (Hallahan et al. 2002). Similarly in TGF β knock out mice, the induction of radiation-induced fibrosis in both skin and lung is inhibited (Martin et al. 2000).
3.2.2 Clinical Observations
The lungs are complex organs containing conducting airways, a complex vascular network, and the alveolar/capillary units where gas exchange occurs. The trachea and proximal airways are lined with pseudostratified ciliated columnar epithelial cells with admixed mucus producing goblet cells. Since these cells have a relatively rapid turnover rate, the mucosa can become acutely denuded during RT. Mild dry cough and/or sore throat during RT is likely related to RT-induced changes in the epithelial lining and decreased clearance of intratracheal debris. Due to the rapid cellular turnover, the depleted mucosa is typically replenished promptly after completing RT and the associated symptoms are transient.
The supporting vascular and other connective tissues are comprised primarily of quiescent “mature” cells with a relatively slow mitotic rate. Although no clinical/pathologic findings are typically observed acutely in these tissues during thoracic RT, late normal tissue injury is possible. For example, bronchial stenosis has been observed following high-dose external beam RT (Miller et al. 2005) as well as brachytherapy (Speiser and Spratling 1993). Similarly, we have seen a case of diffuse pulmonary hypoperfusion after high-dose external beam RT, apparently due to fibrosis of central vascular structures. Months to years following RT, a fibrotic reaction develops within irradiated lung parenchyma. Whether this is a direct effect of RT on the cells comprising the supporting connective tissues, or due to an RT response in other cells, is unclear. While this fibrotic reaction is generally considered to be progressive and irreversible, there is limited experimental data suggesting that this process can be modified and perhaps even reversed (Delanian et al. 1994, 1999).
The more peripheral vascular structures, such as the alveolar capillaries, appear to be more sensitive to RT than the central vasculature. With even modest doses of RT, reductions in regional perfusion, apparently due to sclerosis of these small vessels, has been observed. The extent of reduction in regional lung perfusion has been associated with the degree of change in global lung function as assessed by pulmonary function tests (Fan et al. 2001; Theuws et al. 1998).
A reduction in regional ventilation is also observed after RT, apparently corresponding to a fibrotic/inflammatory reaction within the distal airways. However, the decline in ventilation after RT appears to be less than the corresponding decline in perfusion. Since optimal respiratory function requires both adequate ventilation and perfusion, RT-induced reductions in either will result in ventilation/perfusion mismatch with corresponding decline in overall pulmonary function. That ventilatory and perfusion changes occur in parallel suggests that normal physiologic compensatory mechanisms within both the ventilatory and circulatory systems are contributing to the changes noted after RT.
The histopathological changes observed in the lung after RT are broadly characterized as diffuse alveolar damage. Acutely, increased vascular permeability leads to edema of the interalveolar septa and extravasation of proteinaceous material into the alveoli. Type I pneumocytes are depleted and type II pneumocytes proliferate to restore the integrity of the alveolar epithelium. However, type II pneumocytes are also known to be damaged by RT, leading to the release of surfactant into the alveolar lumen. Increased levels of alveolar surfactant can be seen within hours of RT and can persist for 2–6 weeks (Fleckenstein et al. 2007).
3.2.2.1 Airway Diameter and Airway Resistance
Resistance to airflow within the tracheobronchial tree increases the work of breathing. Assuming laminar flow, the pressure required to produce a given flow rate in a tube is inversely proportional to the fourth power of the radius. This is known as Poiseuille’s law and can be expressed as:
ΔP/V = resistance = 8 nl/πr 4 where ΔP refers to the pressure difference between the two ends of a tube, V is the volume of flow per time period, n is the coefficient of viscosity, l is the length of the tube, and r is the radius of the tube (Comroe 1965). Because the length of the tracheobronchial tree is constant, and the viscosity of air is nearly constant, resistance to airflow is almost entirely dependent on the radius of the airways. Even modest reductions may be sufficient to cause pulmonary symptoms. For example, when the radius of a tube is decreased by a modest 10%, the resistance to airflow is increased by 46%.
Symptomatic narrowing of the tracheobronchial tree is not a common clinical problem after conventional-dose EBRT. However, this has been reported following brachytherapy (Speiser and Spratling 1993) and with higher than conventional doses of external beam RT (≥70 Gy) (Miller et al. 2005; Kelsey et al. 2006). Whether asymptomatic narrowing of the bronchi occurs after conventional doses (60–70 Gy) is not known.
3.2.2.2 Gas Exchange
The rate at which a gas diffuses between the alveoli and capillaries is proportional to the partial pressure gradient of that gas and the available surface area, but inversely proportional to the thickness of the intervening tissues. Disease processes that affect any of these parameters have the potential to limit gaseous exchange. Decreased lung compliance from pulmonary fibrosis or increased airway resistance from bronchial stenosis can decrease the partial pressure of oxygen in the alveoli. Further, RT results in edema of the intraalveolar septa and extravasation of proteinaceous material into the alveoli, both of which negatively affects gas exchange. Later, deposition of scar tissue within the interalveolar septi will impede gas exchange while destruction of alveoli decreases the surface area available for gas exchange.
3.2.2.3 Ventilation and Perfusion
In healthy individuals, alveolar ventilation (V) and pulmonary capillary perfusion (Q) are closely matched for optimal efficiency. For example, if there is decreased alveolar ventilation to a portion of the lung, alveolar and interstitial P 02 will decrease. Local arterioles respond by constricting, diverting blood to better ventilated portions of the lung. On the other hand, if alveolar P 02 rises above normal, local arterioles dilate to maximize oxygen transport. In similar manner, local ventilation is regulated (albeit to a lesser degree than perfusion) in response to local levels of CO2 to optimize the V/Q ratio and maximize the efficiency of gas exchange in the lung.
RT negatively affects both ventilation and perfusion, with perfusion being affected to a greater degree than ventilation (Allavena et al. 1992; Bell et al. 1988). RT reduces the number of perfused alveoli, with a corresponding increase in the alveolar dead-space (i.e., ventilated but under- or nonperfused alveoli). This increased dead-space and reduced functional units leads to a reduction in gas exchange that can cause dyspnea.
4 Clinical Syndromes (Endpoints)
Scoring lung toxicity is difficult for many reasons. For example, a variety of endpoints can be appraised including symptoms, radiographic abnormalities, and pulmonary function tests. These endpoints can be somewhat arbitrarily segregated as reflecting focal versus global, and clinical versus subclinical, changes (Table 3). The SOMA LENT system provides a reasonable way to categorize and grade these various toxicities (Table 4). Each of these endpoints are discussed separately. In broad terms, symptoms occur in approximately 1–40% of patients who receive thoracic RT, depending on the volume of lung irradiated and the dose that is given. Radiologic changes occur in almost all patients if a sensitive imaging tool is used. Changes in PFT’s following RT are common and reflect the competing effects of RT-induced functional loss and recovery of function with tumor regression (for patients with gross intrathoracic lesions).
Table 3
Categorization of representative endpoints for RT-induced lung injury
|
Focal
|
Global
|
|
|---|---|---|
|
Clinical
|
Symptomatic bronchial stenosis
|
Symptoms (dyspnea, cough)
|
|
Subclinical
|
Radiologic abnormalities (computed tomography, perfusion/ventilation scans)
|
Pulmonary function tests; Exercise testing
|
Table 4
LENT SOMA System for Grading Lung Injury
|
Grade 1
|
Grade 2
|
Grade 3
|
Grade 4
|
|
|---|---|---|---|---|
|
Lung
|
||||
|
Subjective
|
||||
|
Cough
|
Occasional
|
Intermittent
|
Persistent
|
Refractory
|
|
Dyspnea
|
Breathless on intense exertion
|
Breathless on mild exertion
|
Breathless at rest, limits all activities
|
Prevents any physical activity
|
|
Chest pain/discomfort
|
Occasional and minimal
|
Intermittent and tolerable
|
Persistent and intense
|
Refractory and excruciating
|
|
Objective
|
||||
|
Pulmonary fibrosis
|
Radiological abnormality
|
Patchy dense abnormalities on radiograph
|
Dense confluent radiographic changes limited to radiation field
|
Dense fibrosis, severe scarring ,and major retraction of normal lung
|
|
Lung function
|
10–25% reduction of respiration volume and/or diffusion capacity
|
>25–50% reduction of respiration volume and/or diffusion capacity
|
>50–75% reduction of respiration volume and/or diffusion capacity
|
>75% reduction of respiration volume and/or diffusion capacity
|
|
Management
|
||||
|
Pain
|
Occasional non-narcotic
|
Regular non-narcotic
|
Regular narcotic
|
Surgical intervention
|
|
Cough
|
Non-narcotic
|
Narcotic, intermittent corticosteroids
|
Respirator, continuous corticosteroids
|
|
|
Dyspnea
|
Occasional O2
|
Continuous O2
|
||
|
Analytic
|
||||
|
PFT
|
Decrease to >75–90% of preTx value
|
Decrease to >50–75% of preTx value
|
Decrease to >25–50% of preTx value
|
Decrease to ≤25% of preTx value
|
|
DLCO
|
Decrease to >75–90% of preTx value
|
Decrease to >50–75% of preTx value
|
Decrease to >25–50% of preTx value
|
Decrease to ≤25% of preTx value
|
|
% O2/CO2 saturation
|
>70% O2, ≤50% CO2
|
>60% O2, ≤60% CO2
|
>50% O2, ≤70% CO2
|
≤50% O2, >70% CO2
|
|
CT/MRI
|
Assessment of lung volume and zones of fibrosis
|
|||
|
Perfusion scan
|
Assessment of pulmonary blood flow and alveolar filling
|
|||
|
Lung lavage
|
Assessment of cells and cytokines
|
|||
4.1 Symptoms
Radiation pneumonitis, typically manifested as shortness of breath, dry cough, and occasionally fever, occurring 1–6 months after treatment (Tsoutsou and Koukourakis 2006), appears to result from an acute inflammatory process within the alveolar spaces. While the radiologic changes associated with this inflammatory reaction are generally limited to the irradiated regions of the lung (Mah et al. 1987; Marks et al. 2000), radiologic changes have been occasionally observed to extend beyond the irradiated field (Gibson et al. 1988; Roberts et al. 1993). Furthermore, bronchoalveolar lavage (BAL) following unilateral irradiation reveals increased lymphocytes from both the irradiated and unirradiated lung (Gibson et al. 1988; Roberts et al. 1993; Martin et al. 1999). These observations suggest that radiation pneumonitis may be type of hypersensitivity pneumonitis (bilateral lymphocytic alveolitis) (Abratt and Morgan 2002). Further, the lymphocytes retrieved with BAL are activated T-lymphocytes (Martin et al. 1999), suggesting that immunologic processes are participating in the radiation reaction. The observation that pneumonitis typically responds well to oral prednisone (Salinas and Winterbauer 1995) also supports the inflammatory/immunologic nature of radiation pneumonitis.
In patients with symptomatic pneumonitis, physical exam is often normal, although rales or a friction rub can sometimes be appreciated. Radiological studies are often, but not always, abnormal. Thus, pneumonitis is a clinical diagnosis. Radiographic abnormalities without symptoms usually do not warrant intervention. Indeed, asymptomatic patients frequency has radiologic findings similar to those seen in symptomatic patients. Thus symptoms and radiologic findings do not necessarily parallel each other. Further, since abnormalities on CT and CXR are very common following RT, reported studies that consider asymptomatic radiologic findings as ‘an event’ likely over-estimate the rate of ‘clinical toxicity’.
Differentiating radiation pneumonitis from other etiologies (e.g., tumor progression, infection, pulmonary emboli, heart disease) can be challenging (Kocak et al. 2005). Ideally, one should be reasonably sure that symptoms suggestive of pneumonitis are not due to these other conditions before therapy is initiated. A short course of antibiotics is occasionally useful if there is concern for infection, with a rapid institution of steroids if symptoms do not respond.
The culminating event in RT-induced lung injury is replacement of normal lung parenchyma with fibrosis, which is often appreciated on radiographic studies but is usually asymptomatic (Marks et al. 2000). However, if the baseline pulmonary reserve of a patient is limited and/or the region of fibrosis is extensive, symptoms can develop. In this circumstance, dyspnea can be progressive and difficult to manage, often requiring long-term steroids, oxygen, and/or rehabilitation. The severity of radiographic abnormalities is not always well-correlated with the presence or severity of pulmonary symptoms (Marks et al. 2000). The time course for the development of RT-associated lung symptoms is shown in Fig. 6a, b.
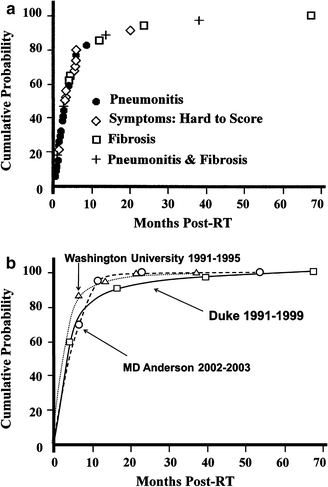

Fig. 6
Time course for the development of RT-associated lung symptoms. a Data from Duke with patients individually noted as having “pneumonitis”, “fibrosis”, as well as cases where the diagnosis was somewhat uncertain due to concurrent illnesses (e.g. infection). b Data from several different centers as shown. (Adapted from Marks et al. 2000)
4.2 Imaging
Multiple radiological studies have been used to assess regional lung injury. The most widely studied include chest X-ray and CT (which evaluate structure and tissue density), and single photon emission computed tomography (SPECT) (which can evaluate the functional endpoints of ventilation and perfusion). The sensitivity of these radiological modalities varies, and thus, the incidence of lung injury depends on the modality utilized. Furthermore, radiological changes are common in patients with lung cancer (who receive relatively large doses to large volumes of lung), and less common in patients with breast cancer or lymphoma (where the doses/volumes are lower). In general, 3D imaging modalities are more sensitive than 2D modalities (CT is more sensitive than CXR), and SPECT-based ventilation/perfusion imaging is more sensitive than either CXR or CT. Comparisons between studies are challenging since the patient populations and RT doses used are variable. Nevertheless, these generalizations appear to be true in studies that have considered multiple imaging modalities (Jackson et al. 2006).
4.2.1 Chest X-ray
The most common finding on plain radiographs after thoracic RT is increased density (radiopacity). This may be secondary to increased interstitial edema and/or alveolar consolidation acutely and pulmonary fibrosis at later time points. Other common findings are nonanatomic margination (corresponding to the RT field edge), volume loss with midline shift, and pleural thickening. Radiographic changes develop shortly after completion of RT, peak at approximately 6 months (Skoczylas et al. 2000; Monson et al. 1998; Lind et al 2000), and typically stabilize by 12 months. However, continued follow-up may show either further progression or even regression in a small number of patients (Skoczylas et al. 2000).
4.2.2 Computed Tomography
The abnormalities noted with CT and chest X-ray are similar. However, CT appears to be more sensitive, in part because paramediastinal abnormalities may be obscured by the cardiac or aortic silhouette on chest X-ray. The typical acute findings are often described as early ground-glass opacities and patchy infiltrates (Fig. 7a). Late findings typically include fibrotic changes with consolidation and volume loss (Fig. 7b). It can sometimes be difficult to differentiate mass-like fibrosis from persistent/recurrent tumor (Koenig et al. 2002). Registration of the planning CT scan (and hence the 3D dose distribution) to the post-RT CT, demonstrates that there are CT-defined increases in tissue density, which are dose-dependent, and largely seen in lung regions receiving ≥40 Gy (Theuws et al. 1998; Kocak and Marks 2005) (Fig. 7e).
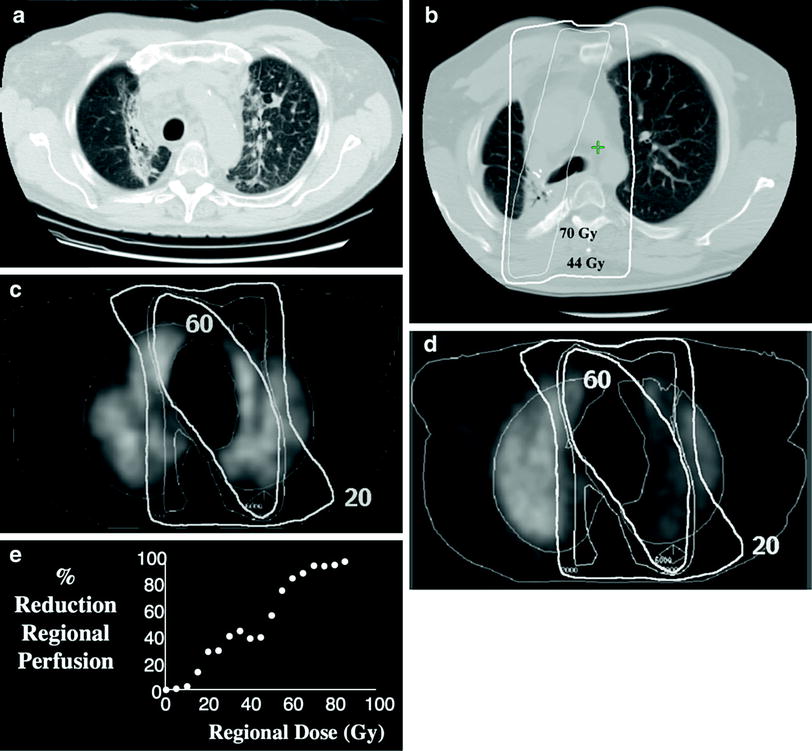

Fig. 7
CT image of a patient 3 months post-RT to the mediastinum for lung cancer. Characteristic increased interstitial markings are seen in the medial lung bilaterally, essentially demarcating the volume of lung that received ≥40 Gy (a). CT image about 2 years post-RT illustrating late fibrotic changes with volume loss. Representative overlying isodose curves shown (b). Transverse SPECT lung perfusion images obtained pre-RT (c) and post-RT (d) in a single patient irradiated for lung cancer. Representative isodose lines are shown. Note the reduction in regional perfusion post-RT. By considering the CT density or perfusion in each pixel independently, and pooling pixels into dose bins, one can create a dose response curve; example panel (e)
Other abnormalities include traction bronchiectasis, diminished pulmonary vascularity, as well as narrowing of the proximal bronchi. Traction bronchiectasis develops from tethering of the bronchial wall by RT-induced fibrosis. Decrease in pulmonary vasculature has been demonstrated outside of the RT field. This maybe secondary to irradiation of central tumors with injury to proximal vessels and/or bronchi (Bell et al. 1988). However, in our studies using SPECT to assess changes in regional perfusion post-RT, we have not typically seen reduced perfusion in unirradiated lung, suggesting that perfusion through larger central vessels is preserved after conventional doses of RT. Symptomatic bronchial stenosis is not commonly observed after conventional doses of RT. With dose escalation, bronchial stenosis has been observed in both symptomatic (Miller et al. 2005) and asymptomatic patients (Kelsey et al. 2006).
4.2.3 Single Photon Emission Computed Tomography
Nuclear medicine imaging of regional perfusion and/or ventilation can also be used to assess regional lung function. Imaging can be planer or 3D (via SPECT). The latter is more sensitive than the former (Bell et al. 1988), analogous to the utility of CT over CXR. Example pre- and post-RT SPECT perfusion images are shown in Fig. 7c, d. Registration of the planning CT scan to pre-and post-RT SPECT scans allows one to quantitatively relate regional RT doses to changes in regional perfusion (Fig. 7e). RT-induced changes in ventilation and perfusion appear to be more common than corresponding changes on CT (Theuws et al. 1998).
Dose-dependent reductions in perfusion are seen as early as 6 weeks after RT (Theuws et al. 2000; Woel et al. 2002), and peaking by approximately 6 months (Woel et al. 2002). Whether recovery occurs with time is unclear. Following modest doses of RT for breast cancer and lymphoma, investigators at the Netherlands Cancer Institute (NKI) report up to 50% recovery by 18 months (Theuws et al. 2000; Boersma et al. 1996). At Duke, we have not seen such recovery, but our study population was predominantly patients with lung cancer receiving higher doses of RT (Woel et al. 2002).
Since the alveolar network is structured in parallel, it is reasonable to hypothesize that changes in global lung function will be related to the extent and severity of regional injury. This is similar to what has been done by surgeons who have tried to relate the percent decline in pulmonary function with the percent of alveoli that are resected (Beckles et al. 2003). We and investigators at the NKI have attempted to relate the sum of the changes in regional perfusion (also termed the integrated response) to changes in pulmonary symptoms and PFTs, with mixed success. While there is a strong correlation between the sum of regional injuries and changes in global function, the correlation coefficients are weak, suggesting that there may be numerous other factors affecting global pulmonary function (Fan et al. 2001; Theuws et al. 1998). The correlation coefficients are higher for the patients with breast and lymphoma than for the patients with lung cancer.
4.2.4 Other Modalities
Magnetic resonance imaging (MRI) has not been widely used to evaluate RT-induced lung toxicity. A preliminary study demonstrated T1 and T2 changes within normal lung parenchyma as early as 17 days after RT. These changes increased over several months before beginning to resolve (Yankelevitz et al. 1994). Another study demonstrated distinct perfusion changes associated with radiation pneumonitis (greater enhancement with both first-pass and redistribution phases) and fibrosis (decreased enhancement with first-pass but greater enhancement with the distribution phase) (Ogasawara et al. 2002). Further MRI investigations may be warranted.
Like MRI, there are few studies evaluating RT-induced lung injury using positron emission tomography (PET). Increased uptake in the lungs of patients with radiation pneumonitis has been reported (Hassaballa et al. 2005; Lin et al. 2000). Another study demonstrated an apparent linear dose response (Guerrero et al. 2007). As expected, there was marked inter-patient variation suggesting an underlying biological susceptibility to injury.
4.3 Pulmonary Function Tests
PFTs objectively assess multiple pulmonary parameters including lung volumes, the amount and rate of air flow (spirometry), and the ability of the lung to transfer gas at the alveolar level. Diffusing capacity of the lung for carbon monoxide (DLCO) is likely the best parameter to assess lung function after RT, since the ultimate role of the lungs is to facilitate gas exchange. Loss of alveolar surface area and thickening of the intraalveolar septi are the primary causes for reductions in DLCO. Thus, both acute (edema, extravasation of material into the alveoli) and chronic (fibrosis, loss of alveoli) injury can affect DLCO. The forced expiratory volume in 1 s (FEV1) is another useful parameter and often interpreted in the context of the FEV1/FVC ratio (FVC = forced vital capacity). A decreased FEV1 with a normal FEV1/FVC ratio is most consistent with a restrictive process, such as fibrosis. A decreased FEV1 and FEV1/FVC ratio is more consistent with an obstructive process.
Assessing the impact of RT on PFTs in patients with lung cancer is challenging. Many patients with lung cancer have baseline pulmonary dysfunction from smoking, such as chronic obstructive pulmonary disease, and some patients continue to smoke after RT with continued accelerated diminutive effects on function. Also, pulmonary function may transiently improve as large central tumors regress. Finally, there is limited data on long-term PFT changes since most patients with lung cancer succumb to their disease within 1–2 years.
With these caveats, most studies show a decline in pulmonary function after RT. Reductions in FEV1 are appreciated approximately 3–6 months post-RT (Table 5), sometimes followed by partial/complete recovery at ≈12 months. This transient decline may be coincident with the acute inflammatory reaction associated with pneumonitis. DLCO typically is reduced to a greater degree than FEV1 (Table 5), and many studies appears to show less recovery at ≈12 months than FEV1. Most studies show a decline in both FEV1 and FVC (Borst et al. 2005; Miller et al. 2003), such that the FEV1/FVC ratio remains in the normal range (Myers et al. 2005), consistent with a restrictive process. In the few patients with long-term changes in PFTs systematically studied beyond one year, there appears to be long-term continued diminution in PFTs 2–8 years post-RT (Miller et al. 2003).
Table 5
Prospective studies evaluating PFTs in patients with lung cancer
|
Study
|
n
|
% Decline in FEV1 after RT (months)
|
||||
|---|---|---|---|---|---|---|
|
3
|
6
|
12
|
18
|
36
|
||
|
Miller et al. (2003)
|
13
|
111
|
0
|
14
|
||
|
Borst et al. (2005)
|
34
|
5
|
8.8
|
13.4
|
||
|
Choi and Kanarek (1994)
|
45a
|
19
|
19b
|
|||
|
Abratt and Willcox (1995)
|
42
|
2
|
+3
|
|||
|
% Decline in DLCO after RT (months)
|
||||||
|
3
|
6
|
12
|
18
|
36
|
||
|
Miller et al. (2003)
|
13
|
8
|
10
|
17
|
||
|
Borst et al. (2005)c
|
34
|
8b
|
14b
|
20b
|
||
|
Choi and Kanarek (1994)
|
45a
|
27
|
||||
|
Abratt and Willcox (1995)
|
42
|
13
|
14
|
|||
Multiple studies, primarily in children, have confirmed that pulmonary function tests are abnormal after whole lung irradiation (Benoist et al. 1982; Ellis et al. 1992; Littman et al. 1976; Miller et al. 1986; Weiner et al. 2006). In general, these studies demonstrate decreased lung volumes (vital capacity, total lung capacity), impaired air flow (forced expiratory volume in 1 second), and perhaps diminished diffusing capacity (DLCO). Multiple factors likely play a role, including impaired chest wall growth, a reduction in the number of functioning alveolar units, and fibrotic changes within the lung. Whether function improves over time (Ellis et al. 1992) or continues to deteriorate (Weiner et al. 2006) is unclear.
5 Radiation Tolerance and Predicting Radiation-Induced Lung Injury
5.1 Whole Lung Irradiation
Irradiation of both lungs occurs in multiple clinical contexts. This includes total body irradiation (TBI) as part of the preparative regimen for stem cell transplants, hemibody irradiation in the setting of diffuse symptomatic metastatic disease, and whole lung irradiation for pulmonary metastases from various malignancies (not commonly done recently). Clinical experience in these settings has permitted the tolerance of whole lung irradiation to be estimated.
5.1.1 Single Fraction (Total Body RT and Hemibody RT)
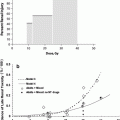
With single fraction whole lung irradiation, the risk of interstitial pneumonitis is primarily dependent on total dose and dose rate. For low-dose rate TBI (5–10 cGy/min), assuming lung density corrections, an early study concluded that the threshold dose for pneumonitis is approximately 9 Gy, the TD5 (dose resulting in a 5% complication rate) is approximately 10 Gy, with a TD50 (dose resulting in a 50% complication rate) of 11–12 Gy (Keane et al. 1981). When higher dose rates are utilized, principally for hemibody irradiation, the dose response curve is shifted to the left. In this circumstance, the threshold dose was estimated to be approximately 7 Gy, with a TD5 of 8 Gy and a TD50 of approximately 9.3 Gy (Keane et al. 1981). In a more recent, comprehensive review, the TD5 (with cyclophosphamide) was estimated to be much lower, approximately 5 Gy for low-dose rate TBI (Sampath et al. 2005). Both studies demonstrated that the dose response curve for whole lung irradiation is steep such that a difference of only 1–2 Gy increases the risk of pneumonitis significantly (Fig. 8) The development of pneumonitis after whole lung irradiation is an ominous sign, proving fatal in up to 80% of patients (Fryer et al. 1978). The difference in mortality for similar doses between TBI and HBI dose response appears to have been due to the low-dose rate protraction for TBI. Decreasing the dose rate from 0.5 to 0.1 by per minute decreases the incidence of pneumonitis associated with whole lung irradiation during TBI. This was a byproduct of greater distance from the radiation source, required to produce a large field to encompass the whole body.
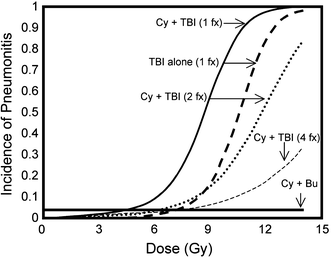

Fig. 8
The risk of pneumonitis following whole lung RT as part of TBI. The addition of chemotherapy appears to move the dose response curve to the left, and fractionating the RT moves the dose response curve to the right (Adopted from QUANTEC, Marks et al. 2010, IJROBP)
5.1.2 Multiple Fractions
Two randomized studies treated patients with osteogenic sarcoma with prophylactic, fractionated whole lung irradiation. A Mayo clinic trial treated patients with 15 Gy over 2 weeks with concurrent actinomycin D (Rab et al. 1976). A European study treated patients with 17.5 Gy in 2 weeks without chemotherapy (Breur et al. 1978). Lung density corrections were not utilized in either study, and thus the true total physical doses delivered were likely in the neighborhood of 17 and 20 Gy in these two studies, respectively. No cases of pneumonitis were reported in either trial. A retrospective study of whole lung irradiation for a variety of tumor histologies, mostly pediatric, reported no cases of pneumonitis after doses ranging from 15–25 Gy (Newton and Spittle 1969). Furthermore, in several retrospective studies in which TBI was utilized in the preparative regimen prior to stem cell transplant, the risk of interstitial pneumonitis appeared to be lower with fractionation (Cosset et al. 1989; Pino et al. 1982; Shank et al. 1983). These studies suggest that fractionation increases lung tolerance. However, in two randomized studies comparing single fraction TBI with fractionated TBI, there was no difference in the risk of interstitial pneumonitis (virus + idiopathic) (Table 6). There were less cases of idiopathic pneumonitis with fractionation (Deeg et al. 1986; Girinsky et al. 2000).
Table 6
Randomized studies of TBI fractionation for hematological malignancies
|
Author
|
n
|
Dose/fractions
|
Dose rate
|
Chemotherapy
|
Pneumonitis rate
|
|---|---|---|---|---|---|
|
Girinsky et al. (2000)
|
147
|
10 Gy/1a
14.85 Gy/11b
|
12.5 cGy/min
25 cGy/min
|
CYC or MEL
CYC or MEL
|
19% (6 mos)
14% (6 mos)
|
|
Deeg et al. (1986)
|
53
|
10 Gy/1
12 Gy/6
|
6 cGy/min
6 cGy/min
|
CYC
CYC
|
26% (crude)
23% (crude)
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree