, Leonid Tiutin2 and Thomas Schwarz3
(1)
Russian Research Center for Radiology and Surgery, St. Petersburg, Russia
(2)
Department of Radiology and Nuclear Medicine, Russian Research Center for Radiology, St. Petersburg, Russia
(3)
Department of Nuclear Medicine Division of Radiology, Medical University Graz, Graz, Austria
Abstract
Timely diagnosis of lung cancer (LC) is difficult in the stage in which radical treatment is possible, due to scarce clinical manifestations. Numerous symptoms usually appear only as the tumor progresses (Zyryanov et al. 1997). Clinical manifestations of LC depend on three main factors: clinical-anatomic form of the disease, presence and localization of distant metastases as well as system disturbances caused by paraneoplastic syndromes. For example, central LC is characterized by cough, hemoptysis, temperature rise, shortage of breath, fever and productive cough as a consequence of paracancerous pneumonitis. In peripheral cancer, pain in the thorax, cough, shortage of breath and symptoms of lung abscess (in case of tumor necrosis) come to the foreground.
Timely diagnosis of lung cancer (LC) is difficult in the stage in which radical treatment is possible, due to scarce clinical manifestations. Numerous symptoms usually appear only as the tumor progresses (Zyryanov et al. 1997). Clinical manifestations of LC depend on three main factors: clinical-anatomic form of the disease, presence and localization of distant metastases as well as system disturbances caused by paraneoplastic syndromes. For example, central LC is characterized by cough, hemoptysis, temperature rise, shortage of breath, fever and productive cough as a consequence of paracancerous pneumonitis. In peripheral cancer, pain in the thorax, cough, shortage of breath and symptoms of lung abscess (in case of tumor necrosis) come to the foreground.
5.1 Pathologic Anatomy and Mechanisms of Metastases
Presently it is accepted to use the clinical-anatomical and pathomorphological classifications to characterize diverse forms of LC (Tyulyandin and Moiseenko 2004).
5.1.1 Clinical-Anatomical Classification of LCs
Central Cancer of the Lung
Endobronchial
Peribronchial nodal
Branched
Peripheral Cancer of the Lung
Odal
Pneumonia-like (bronchioloalveolar)
Cancer of the lung apex (Pancoast syndrome)
5.1.2 Histological Classification of the WHO (1981)
Dysplasia/carcinoma in situ
Planocellular cancer (30%)
Small cell cancer (18.2%): oat cell cancer, transitional cell cancer
Adenocarcinoma (30.7%): acinar, papillary, bronchioloalveolar, solid cancer with mucin formation
Large cell cancer (9.4%): giant cell cancer, clear cell cancer
Glandular-planocellular (1.5%)
Carcinoid tumors (1.0%)
Cancer of the bronchial glands (9.2%): mucoepidermoid, adenocystic and others
In 52% of cases the malignant neoplasm is situated in the right lung, and in 48% of cases it is in the left lung. The spread of LC occurs in three ways: lymphogenous, hematogenous and implantation. Among regional lymph nodes, the intrathoracic (mediastinal and intrapulmonary), prescalenic and supraclavicular nodes are the first to be tumor-involved. In case of hematogenic metastasis, the brain, bones, suprarenal glands, contralateral lung, liver, pericardium and kidneys are affected. The implantation spread manifests itself with lesions of the pleura with development of carcinomatosis and cancer pleuritis.
5.2 Methods of Diagnosis
The detection of LC is possible at screening and additional examinations. The widest-spread kind of screening examination is prophylactic fluorography. It is meant to reveal a symptom group whose nature is specified at further additional patient examination. The algorithm of additional patient examination consists of four stages:
1.
The preliminary stage consists in doing survey (diagnostic) fluorography or roentgenography of the thoracic organs in frontal and lateral projections.
2.
The tomographic stage includes performing MSCT in order to examine tumor dissemination.
3.
The bronchologic stage consists in cytological examination of phlegm and in fiberoptic bronchoscopy.
4.
The differential diagnosis stage includes roentgenoscopy, transthoracal puncture (aspiration) biopsy, bronchography, bronchoscopy, angiography, thoracoscopy, mediastinoscopy, MRI, diagnostic thoracotomy.
It should be noted that despite the wide spectrum of modern methods of clinical diagnosis and radiodiagnosis, the problem of differential diagnosis of solitary lung masses (SLMs) is still unresolved. SLM is a node in the pulmonary parenchyma with diameter less than 3 cm, which has clear boundaries, a round or oval form. Every year up to 13,000 new cases of SLM are detected. Additionally, 50–60% of patients are subsequently diagnosed with a malignant lung lesion. In the remaining cases, the pathologic process is based on benign granulomas, local pneumofibrosis as well as hamartomas and alterations conditioned by tubercular process. The correct assessment of clinical-radiological data in patients of this category will permit unnecessary surgery to be avoided. Currently, when SLM is detected, especially in young patients, expectant management is adopted. At the same time, according to Yankelevits et al. (1997), absence of growth of pathologic nodes only in 65% of cases suggests the benign character of changes.
5.2.1 PET Diagnosis
The role of PET in LC diagnosis is ambiguous. On the one hand, the method is highly sensitive and permits visualization of malignant neoplasms of the lung. On the other hand, the nature of detected changes cannot always be defined by means of 18F-FDG PET. The main cause of diagnostic problems is the absence of pathognomonic PET signs permitting discrimination of tumor lesions from inflammatory changes (Baum et al. 2002). For example, according to Gould et al. (2001) who implemented analysis of 1474 SLM cases, the sensitivity of the method was 96%, its specificity being only 74%. The results of other researchers indicate that the sensitivity of the method ranges within 82–100% and its specificity varies from 63% to 90% (Bury and Rigo 2000; Kubota et al. 1990; Lowe et al. 1994; Partz et al. 1994) A diagnostic advantage of PET/CT versus PET in diagnosing lung cancer is not clearly proofed. Metaanalyses of the rare papers indicate an increase of sensitivity and decrease of specificity (woolley 2008).
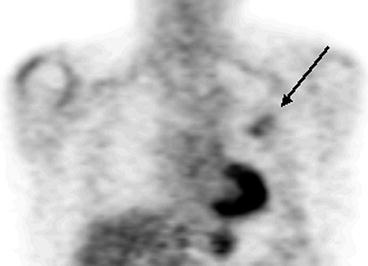
The widest spread cause of false-negative results in PET examination is the small size of formation (less than 5 mm). At the same time, according to Herber et al. (2004), the efficiency of PET in diagnosis of solid lung masses with size up to 1 cm is higher than that of CT. They report that PET sensitivity makes up 93%, its specificity is 77%, positive prognostic value is 72% and negative prognostic value is 94%. Another cause of false-negative results is isometabolism of 18F-FDG in cases of some histological tumor types. For example, bronchoalveolar tumor (BAT), mucinous adenocarcinoma, carcinoid masses and metastases of thyroid cancer and of renal cell cancer may not be diagnosed with help of PET. The absence of pathological radiopharmaceutical (RP) accumulation in malignant neoplasm can be caused by the high differentiation of cell structures and consequently by the low biological activity of tumor. This is why negative PET results in LC patients usually imply a favorable disease prognosis (Schiepers et al. 2002). However, it should be noted that in most cases the absence of pathological 18F-FDG uptake in the projection of a solid mass visualized in X-ray denotes a benign process (Mavi et al. 2005; Vansteenkiste et al. 2004). According to Lowe et al. (1994), only 5% of patients with negative PET results are subsequently diagnosed with LC. In connection with these data the authors conclude that the accuracy of the method in differential diagnosis of LC, benign neoplasms and chronic inflammatory diseases in the remission stage may reach 95% (Fig. 5.1).
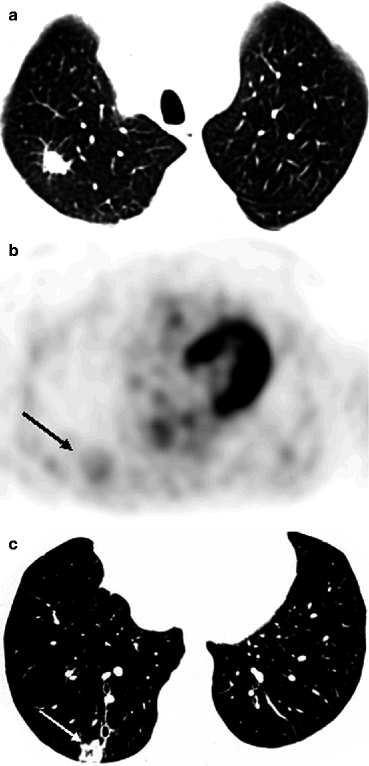
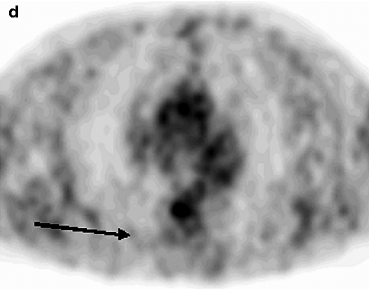


Fig. 5.1
MSCT and PET data in patients with pneumosclerosis (a, b) and thromboembolism of small pulmonary artery branches of the basal segments of the right lung (c, d)
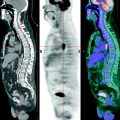
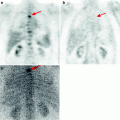
The analysis of scientific literature of the last years shows that there are many works concerning the pathophysiological characteristics of high 18F-FDG uptake in the area of inflammation. It has been proved that standardized uptake values (SUVs) in acute and chronic infectious states range within large limits. In some cases the intensity of RP accumulation in tumor can be lower than in inflammation. Most often such a situation is observed in granulomatous lesion of pulmonary parenchyma (Fig. 5.2).
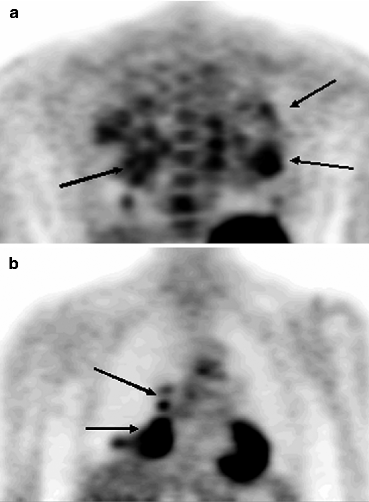

Fig. 5.2
PET results in a patient with generalized sarcoidosis (a) and in a patient with tuberculosis of the right lung (b)
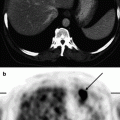
In pneumonia of bacterial or viral genesis, SUVs are not usually high (Fig. 5.3). However, it should be taken into account that low SUVs may be detected in some histological types of malignant tumors (Fig. 5.4). Small-sized masses are also characterized by low SUV values in consequence of the volume averaging of the intensity of RP accumulation since absolute 18F-FDG accumulation is observed in the area of minimum spatial resolution.