MR imaging has emerged as a major new research and diagnostic tool for various pulmonary diseases, especially lung cancer. State-of-the art thoracic MR imaging now has the potential to be used as a substitute for traditional imaging techniques and/or to play a complementary role in patient management. This article focuses on these recent advances in MR imaging for lung cancer imaging, especially for pulmonary nodule assessment, lung cancer staging, postoperative lung function prediction, and prediction and evaluation of therapeutic response and recurrence. The potential and limitations of routine clinical application of these advances are discussed and compared with those of other modalities.
Key points
- •
Non–contrast enhanced (CE) MR imaging techniques can improve differentiation of malignant from benign nodules compared with routine clinical protocols.
- •
Dynamic MR imaging with three-dimensional GRE sequence and ultrashort TE has the potential to play a complementary role in the characterization of pulmonary nodules and as a viable alternative to dynamic CE CT, FDG-PET, and/or PET/CT.
- •
Because multidetector row CT is widely used clinically, potential use of MR imaging in T-factor assessment may be limited; when MR imaging is used in this setting, STIR turbo SE imaging and three-dimensional CE GRE sequence may be helpful.
- •
For N-factor assessment, STIR turbo SE imaging can improve diagnostic performance compared with DWI and FDG-PET and/or PET/CT, although DWI is considered at least as valuable as PET or PET/CT.
- •
For M-factor assessment, whole-body MR imaging with and without DWI is as effective as FDG-PET or PET/CT in routine clinical practice.
Outline
Since the 1980s many physicists and radiologists have been trying to evaluate the efficacy of MR imaging for various lung diseases, and for mediastinal and pleural diseases. Until 2000, however, thoracic MR imaging had shown limited clinical relevance, and could not be substituted for computed tomography (CT) and other modalities. It was therefore generally used for select clinical indications. During the first decade of this century, numerous basic and clinical researchers in radiology reported technical advances in sequencing, scanners and coils, adoption of parallel imaging techniques, use of contrast media, and development of postprocessing tools. In addition, several promising new techniques, including short inversion time inversion recovery (STIR) turbo spin echo (SE) imaging, diffusion-weighted imaging (DWI), and pulmonary functional MR imaging, have been extensively researched and developed, leading to a re-examination of MR imaging as a new research and diagnostic tool for various pulmonary diseases, especially lung cancer. As a result, state-of-the-art thoracic MR imaging now has the potential to be used as a substitute for traditional imaging techniques and/or to play a complementary role in patient management.
This article focuses on these recent advances in MR imaging for lung cancer imaging, especially for pulmonary nodule assessment, lung cancer staging, prediction of postoperative lung function, prediction of therapeutic response, and recurrence. The potential and limitations for routine clinical application of these advances are also discussed and compared with those of other modalities, such as CT, PET, and PET/CT.
Outline
Since the 1980s many physicists and radiologists have been trying to evaluate the efficacy of MR imaging for various lung diseases, and for mediastinal and pleural diseases. Until 2000, however, thoracic MR imaging had shown limited clinical relevance, and could not be substituted for computed tomography (CT) and other modalities. It was therefore generally used for select clinical indications. During the first decade of this century, numerous basic and clinical researchers in radiology reported technical advances in sequencing, scanners and coils, adoption of parallel imaging techniques, use of contrast media, and development of postprocessing tools. In addition, several promising new techniques, including short inversion time inversion recovery (STIR) turbo spin echo (SE) imaging, diffusion-weighted imaging (DWI), and pulmonary functional MR imaging, have been extensively researched and developed, leading to a re-examination of MR imaging as a new research and diagnostic tool for various pulmonary diseases, especially lung cancer. As a result, state-of-the-art thoracic MR imaging now has the potential to be used as a substitute for traditional imaging techniques and/or to play a complementary role in patient management.
This article focuses on these recent advances in MR imaging for lung cancer imaging, especially for pulmonary nodule assessment, lung cancer staging, prediction of postoperative lung function, prediction of therapeutic response, and recurrence. The potential and limitations for routine clinical application of these advances are also discussed and compared with those of other modalities, such as CT, PET, and PET/CT.
Introduction
Lung cancer is one of the most common cancers worldwide with the highest mortality for men and women, and frequently manifests as a lung nodule or mass. Radiologically identified lung lesions that are less than 30 mm in diameter are known as pulmonary nodules, whereas those larger than 30 mm are called masses. Diagnosis and further characterization of pulmonary nodules is one of the most important areas of pulmonary medicine for appropriate management. Early diagnosis is important because non–small cell lung cancer (NSCLC), which currently accounts for 80% of all lung cancers, can be cured surgically if detected at an early stage. However, most patients with NSCLC present late in the course of their illness, often at an inoperable, advanced stage.
Apart from characterization, another potential application of MR imaging is tumor staging. NSCLC is usually staged with the tumor, lymph node, and metastasis (TNM) staging system, which has been recommended by the Union Internationale Contre le Cancer, the American Joint Committee on Cancer, and the International Association for the Study of Lung Cancer. However, small cell lung carcinoma, which constitutes approximately 13% to 20% of all lung cancers, is staged with a two-stage system developed by the Veteran’s Administration Lung Cancer study group. Irrespective of the type of staging system, radiologic examinations including CT, MR imaging, PET with 2-[fluorine-18] fluoro-2-deoxy- d -glucose (FDG-PET), and FDG-PET combined with CT (FDG-PET/CT) are important for pretherapeutic assessment in conjunction with pathologic information from transbronchial/CT-guided/mediastinoscopic biopsies.
Recent technical advances in MR systems, application of parallel imaging, introduction of new MR techniques, and use of contrast media have markedly improved the diagnostic accuracy of MR imaging in this setting. Also, FDG-PET combined or fused with MR imaging (FDG-PET/MR imaging) is now being evaluated in several studies as an emerging technique. This article focuses on these recent advances in MR imaging, and its potential clinical applications and limitations particularly in pulmonary nodule assessment and lung cancer staging.
Pulmonary nodule and mass assessment
Most solitary pulmonary nodules are incidental findings on chest radiographs obtained for unrelated diagnostic work-ups. In addition, a growing number are being detected on chest CTs as a direct result of increased use of CT examinations in routine clinical practice. Results of national lung cancer screening trials and previous CT-based lung cancer screening studies have led to a growing need for management of pulmonary nodules. Although CT facilitates detection of an unsuspected lung cancer, the major drawback is exposure to ionizing radiation and a concomitant increase in the detection of benign and indeterminate nodules. This upsurge in the frequency of nodule detection is associated with an increase in the number of follow-up studies, further radiologic examinations, and/or of interventional procedures and associated costs. MR imaging is associated with no radiation, and is being evaluated as a possible alternative or complementary technique for management of such nodules in addition to traditionally used techniques.
Since the clinical application of MR imaging in the chest, SE or turbo SE sequences have aided in the detection of numerous pulmonary nodules including lung cancers, pulmonary metastases, and low-grade malignancies, such as carcinoids and lymphomas. Such lesions usually show low or intermediate signal intensity on T1-weighted imaging (T1WI) and slightly high intensity on T2-weighted imaging (T2WI). Previous reports have suggested that T2WI and pre– and post–contrast-enhanced (CE) T1WI are useful for diagnosis of specific types of nodule or masses, such as bronchoceles, tuberculomas, mucin-containing tumors, hamartomas, and aspergillomas based on their specific MR findings, although significant overlap occurs between benign and malignant nodules or masses.
To overcome this limitation, STIR turbo SE imaging and DWI were introduced in 2008 as promising sequences for nodule assessment. Koyama and colleagues demonstrated that STIR turbo SE imaging was significantly better than non-CE T1WI or T2WI for differentiating malignant from benign solitary pulmonary nodules, with sensitivity, specificity, and accuracy of 83.3%, 60.6%, and 74.5%, respectively. DWI involves estimation of apparent diffusion coefficient (ADC), which evaluates the diffusivity of water molecules within tissue for b values ranging from 0 s/mm 2 to a maximum value of 500 s/mm 2 to 1000 s/mm 2 in routine clinical practice. With ADC evaluation, the range of sensitivity and specificity of DWI in this setting has been reported to be 70.0% to 88.9% and 61.1% to 97.0%, respectively. One study evaluated signal intensity of lesion to spinal cord ratio at high b value DWI and suggested that this parameter is more useful than ADC. The authors found lesion to spinal cord ratio to have a sensitivity, specificity, and accuracy of 83.3%, 90.0%, and 85.7%, respectively, and noted that the accuracy of this new parameter was significantly higher than that of ADC (85.7% vs 50.0%). It would therefore be advantageous to use the previously mentioned non-CE MR imaging techniques (STIR turbo SE imaging and DWI) in routine clinical protocols along with other sequences to improve differentiation between malignant and benign nodules. This approach can be considered at least as efficacious as FDG-PET or PET/CT ( Figs. 1 and 2 ).
In addition to the previously mentioned non-CE MR imaging techniques, dynamic CE MR imaging can lead to further improvement of diagnostic performance. Since dynamic CE MR imaging was first reported, various MR imaging techniques have been used for this purpose, and have evolved with improvements in MR imaging systems, sequences, and the use of injectors and development of new software. There are currently three major methods for dynamic MR imaging of the lung. These broad categories include two-dimensional SE or turbo SE sequences and various types of two-dimensional and three-dimensional gradient-echo (GRE) sequences. It is recommended that the enhancement patterns within nodules and/or parameters determined from signal intensity-time course curves be assessed visually. The diagnostic performance of dynamic MR imaging techniques in distinguishing malignant from benign nodules has been reported to have a sensitivity ranging from 94% to 100%, specificity of 70% to 96%, and accuracy of more than 94%. In addition, a meta-analysis found that there were no significant differences in diagnostic performance of dynamic CE CT, dynamic CE MR imaging, FDG-PET, and single-photon emission tomography (SPECT), although dynamic MR imaging with the three-dimensional GRE sequence and ultrashort echo time (TE) proved to be superior in a direct and prospective comparison study of dynamic CE CT and coregistered FDG-PET/CT (see Figs. 1 and 2 ). The use of dynamic MR imaging with the three-dimensional GRE sequence and ultrashort TE is therefore more likely to be effective than other methods and may lead to improved diagnostic performance of dynamic CE MR imaging. This method has the potential to play a complementary role in the characterization of pulmonary nodules and as a viable alternative to dynamic CE CT, FDG-PET, and/or PET/CT.
Lung cancer staging
TNM classification is essential for lung cancer management, and it is hoped that this classification be as accurate as possible to direct appropriate management. Currently, thin-section CE CT is deemed effective for assessment of tumor extent because of its multiplanar capability and higher spatial resolution, whereas FDG-PET and PET/CT are used for accurate diagnosis of metastatic lymph nodes and distant metastatic sites except brain metastasis. In contrast to the previously mentioned modalities, MR imaging has been used in limited scenarios since 1991. With recent advances in MR imaging techniques, it is now possible to improve TNM staging accuracy in patients with lung cancer. In this section, historical, traditional, and newly applied MR imaging techniques for TNM staging are introduced and discussed in the hope that they will help improve imaging protocols for patients with lung cancer in daily clinical practice.
T-Factor Assessment on MR Imaging
In relation to T-factor assessment, the Radiologic Diagnostic Oncology Group published a comparative study of CT and non-CE MR imaging for TNM staging of 170 patients with NSCLC in 1991. This study found that operability assessment based on differentiating T3-T4 from T1-T2 tumors on non-CE MR imaging (sensitivity, 56.0%; specificity, 80.0%) was not significantly different from that of CT (sensitivity, 63.0%; specificity, 84.0%).
New MR imaging techniques have been added to improve MR imaging–based T-factor assessment since 1997. Sakai and colleagues used dynamic cine MR imaging during breathing rather than static MR imaging, resulting in improved diagnosis of chest wall invasion. This study assessed the movement of the tumor along the parietal pleura during the respiratory cycle displayed as a cine loop in a manner similar to that used for dynamic expiratory multisection CT. In another study, the sensitivity, specificity, and accuracy of dynamic cine MR imaging for the detection of chest wall invasion were 100%, 70%, and 76%, respectively, whereas those of conventional CT and static MR imaging were 80%, 65%, and 68%. Dynamic cine MR imaging in conjunction with static MR imaging can therefore be considered a useful method for chest wall invasion assessment.
Another promising technique, first reported in 2001, is CE MR angiography for assessment of cardiovascular or mediastinal invasion. In comparison with CE CT and cardiac-gated T1WI, it has a higher sensitivity, specificity, and accuracy for detection of mediastinal and hilar invasion ranging from 78% to 90%, 73% to 87%, and 75% to 88%, respectively. CE MR angiography based on three-dimensional CE GRE sequence can thus help improve the diagnostic capability of MR imaging for T-factor assessment in routine clinical practice.
N-Factor Assessment on MR Imaging
The performance of MR imaging for N-factor assessment was previously not considered to be significantly different from CT because diagnostic criteria for differentiation of metastatic from nonmetastatic lymph nodes primarily depended on lymph node size. Therefore, in previous publications, the multiplanar capability of MR imaging was regarded as the only advantage in the detection of lymph nodes in such areas as the aortopulmonary window and subcarinal regions, which were difficult to assess on the commonly used axial images in CT.
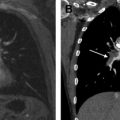
To improve the diagnostic performance of N-factor assessment on MR imaging, cardiac- and/or respiratory-triggered conventional or black-blood STIR turbo SE imaging has been tested since 2002, and has demonstrated its superiority over CE CT, FDG-PET, or PET/CT and other MR imaging sequences. STIR turbo SE imaging consists of a simple sequence that is easily incorporated into clinical protocols to yield T1 and T2 relaxation times. On STIR turbo SE images, metastatic lymph nodes show high signal intensity and nonmetastatic lymph nodes demonstrate low signal intensity ( Fig. 3 ).
According to previously published results, sensitivity of quantitatively and qualitatively assessed STIR turbo SE imaging ranged, on a per-patient basis, from 83.7% to 100.0%, specificity from 75.0% to 93.1%, and accuracy from 86.0% to 92.2%, and these values were equal to or higher than those for CE CT, FDG-PET, or PET/CT. Yet another study showed that the quantitative and qualitative sensitivity, specificity, and accuracy of STIR turbo SE imaging were not significantly different from FDG-PET/CT. However, the combination of FDG-PET/CT with STIR turbo SE imaging was found to be significantly more effective in detecting nodal involvement on a per-patient basis (96.9% specificity, 90.3% accuracy) than FDG-PET/CT alone (65.6% specificity, 81.7% accuracy).
In 2008, DWI was introduced as another promising MR imaging technique for this purpose. Sensitivity, specificity, and accuracy of DWI reportedly range, on a per-patient basis, from 77.4% to 80.0%, 84.4% to 97.0%, and 89.0% to 95.0%, respectively, and these results seem to be similar to or better than those for FDG-PET or PET/CT (see Fig. 3 ). Ohno and colleagues prospectively and directly compared these modalities to determine the clinical relevance of MR imaging–based N-factor assessment compared with FDG-PET/CT. In this study, sensitivity and/or accuracy of STIR turbo SE imaging (quantitative sensitivity, 82.8%; qualitative sensitivity, 77.4%; quantitative accuracy, 86.8%) proved to be significantly higher than those of DWI (74.2%, 71.0%, and 84.4%, respectively) and FDG-PET/CT (quantitative sensitivity, 74.2%). This means that quantitative and qualitative assessment of the N stage of patients with NSCLC obtained with STIR turbo SE MR imaging is more sensitive and more accurate than those obtained with DWI and FDG-PET/CT. According to these results and considering the limitations of DWI and FDG-PET/CT for detection of small metastatic foci or lymph nodes, STIR turbo SE imaging may be the better MR imaging technique to use before surgical treatment or lymph node sampling for accurate pathologic TNM staging and after treatment. Further technical improvements in DWI are needed to overcome its current limitations and enable it to function in a complementary role to STIR turbo SE imaging in routine clinical practice.
M-Factor Assessment on MR Imaging
M-factor assessment for detection of metastasis in patients with lung cancer has major implications for management and prognosis, and an accurate diagnosis in this respect may help clinicians provide the most appropriate treatment and/or management for patients with lung cancer. For M-factor assessment, intrathoracic and extrathoracic metastases have to be evaluated. For this purpose, chest radiography, CT, MR imaging, bone scintigraphy, and PET or PET/CT are used in routine clinical practice. When MR imaging is used in this setting, a clear understanding of the diagnostic performance of state-of-the-art MR imaging for intrathoracic and extrathoracic metastases assessment is necessary.
Intrathoracic metastasis assessment
Chest radiography and CT are often regarded as the first choice for assessment of intrathoracic metastasis; other commonly used modalities include PET/CT. Since 1992, several investigators have tried to determine the capability of MR imaging in evaluating metastatic lung nodules in the context of primary lung cancer or other primary malignancies by using various sequences with 1.5- and 3.0-T scanners. In addition, combination of the previously mentioned MR imaging techniques to differentiate malignant nodules (metastatic and primary malignancies) from benign nodules has been evaluated. The detection rate or sensitivity of MR imaging using various sequences on 1.5- and 3.0-T systems has ranged from 36.0% to 96.0%. The study with the largest population to date compared CT with 1.5-T MR imaging and demonstrated that although the overall detection rate of thin-section CT (97%) was superior to that of respiratory-triggered STIR turbo SE imaging (82.5%), there was no significant difference between the two in the detection rate for all types of malignant nodules. Therefore, the currently available MR imaging technique can have a complementary role in intrathoracic metastasis detection. More importantly, these findings emphasize the need to carefully check for nodules on thoracic MR images of oncology patients.
Extrathoracic metastasis assessment
For evaluation of extrathoracic metastases, CE CT, bone scintigraphy, brain CE MR imaging, and PET or PET/CT have been used in routine clinical practice in accordance with published guidelines or recommendations. Whole-body MR imaging has become clinically feasible after the introduction of fast imaging and moving table equipment. Since 2007, it has been regarded as a single, cost-effective imaging test using 1.5- and 3-T systems for patients with not only lung cancer, but also other malignancies. Furthermore, whole-body DWI has been recommended as a promising new tool for whole-body MR imaging examination of oncologic patients. Comparison of the diagnostic performance of whole-body MR imaging for M-factor assessment with FDG-PET or PET/CT has shown that the diagnostic capability of whole-body MR imaging with or without DWI (sensitivity, 52.0%–80.0%; specificity, 74.3%–94.0%; accuracy, 80.0%–87.7%) was equal to or significantly higher than that of FDG-PET or PET/CT (sensitivity, 48.0%–80.0%; specificity, 74.3%–96%; accuracy, 73.3%–88.2%). However, one drawback associated with the use of whole-body DWI in this setting needs to be carefully considered. The specificity (87.7%) and accuracy (84.3%) of whole-body DWI alone on a per-patient basis was found to be significantly lower than FDG-PET/CT (specificity, 94.5%; accuracy, 90.4%). Another study found that the diagnostic accuracy of whole-body MR imaging combined with DWI (87.8%) was not significantly different from that of FDG-PET/CT ( Fig. 4 ), although that of whole-body MR imaging without DWI (85.8%) was lower than that of FDG-PET/CT. Therefore, it is advisable to use whole-body DWI as part of whole-body MR imaging examination to improve the diagnostic accuracy of M-factor assessment of patients with NSCLC.