Adenocarcinoma in situ
Minimally invasive adenocarcinoma
Lepidic predominant adenocarcinoma
Predominantly invasive adenocarcinoma with nonmucinous, lepidic component
Invasive mucinous adenocarcinoma
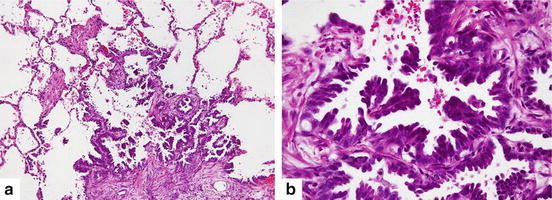
Fig. 7.1
Atypical adenomatous hyperplasia (a) Discrete parenchymal lesion showing alveolar wall thickening with alveolar lining cells proliferation (Hematoxylin and eosin 100×). (b) Cuboidal to columnar pneumocytes with mild to moderate cytological atypia revealing gaps between adjacent cells (Hematoxylin and eosin 200×)
(B)
Minimally invasive adenocarcinoma (MIA)
MIA is a small (≤3 cm) solitary, usually nonmucinous adenocarcinoma with a predominantly lepidic pattern with small foci of invasion (≤5 mm). The invasive component to be measured includes histologic subtypes other than a lepidic pattern or tumor cells infiltrating myofibroblastic stroma. MIA is excluded if the tumor invades lymphatics, blood vessels or pleura, or contains tumor necrosis.
(C)
Invasive adenocarcinoma
Invasive adenocarcinomas are classified by single predominant patterns: lepidic, acinar, papillary, micropapillary and solid (Table7.2, Fig. 7.2).
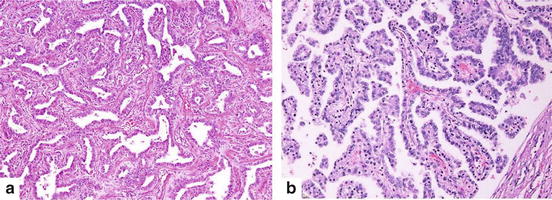
Table 7.2
IASLC/ATS/ERS classification of lung adenocarcinoma in resection specimens
Preinvasive lesions |
Atypical adenomatous hyperplasia |
Adenocarcinoma in situ (≤3 cm, pure lepidic growth without invasion, formerly BAC) |
Nonmucinous |
Mucinous |
Mixed mucinous/nonmucinous |
Minimally invasive adenocarcinoma (≤3 cm lepidic predominant tumor with ≤5 mm invasion) |
Nonmucinous |
Mucinous |
Mixed mucinous/nonmucinous |
Invasive adenocarcinoma |
Lepidic predominant (formerly nonmucinous BAC pattern, with >5 mm invasion) |
Acinar predominant |
Papillary predominant |
Micropapillary predominant |
Solid predominant with mucin production |
Variants of invasive adenocarcinoma |
Invasive mucinous adenocarcinoma (formerly mucinous BAC) |
Colloid |
Fetal (low and high grade) |
Enteric |

Fig. 7.2
(a) Acinar adenocarcinoma consists of round to oval shaped malignant glandular structures with stromal infiltration (Hematoxylin and eosin 100×). (b) Papillary adenocarcinoma composed of papillary proliferation along fibrovascular cores lined by malignant cuboidal to columnar tumor cells (Hematoxylin and eosin 100×)
7.1.6.4 Grading of Adenocarcinoma
No grading system with specific morphologic criteria is established for lung adenocarcinoma. Nevertheless, a three-tier grading scheme is typically used (well, moderate, and poorly differentiated) based on architectural pattern and nuclear atypia. In the case of more than one grade in a tumor, the overall grade is determined by the component with the least differentiation [54]. Currently, the association between prognosis and pattern is reported as follows; poor (solid and micropapillary), favorable (nonmucinous lepidic), and intermediate (papillary and acinar) [52].
7.1.6.5 Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) is a malignant epithelial tumor arising from bronchial epithelial cells with keratinization and/or intercellular bridge. Several variants are mentioned in the 2004 WHO classification.
7.1.6.6 Gross Pathology
Most SCCs are centrally located with white to gray discoloration depending on the extent of fibrosis. Large peripheral SCCs often display necrosis and cavitations. Central tumors usually show intraluminal polypoid growth and may occlude the bronchial lumen. Bronchiectasis, atelectasis, and infective bronchopneumonia are frequently observed in the lung distal to the obstruction.
7.1.6.7 Histopathology
SCC is characterized by keratinization, pearl formation, and intercellular bridges. These features vary with degree of differentiation, being prominent in well-differentiated tumors and focal in poorly differentiated tumors [54] (Fig. 7.3).
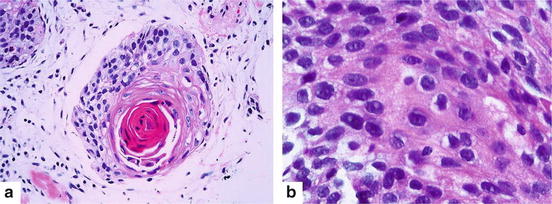

Fig. 7.3
Squamous cell carcinoma (a) Keratin pearl is an evidence of squamous cell differentiation (Hematoxylin and eosin 200×). (b) Intercellular bridges are also a characteristic manifestation (Hematoxylin and eosin 400×)
IHC is valuable in the distinction of pulmonary adenocarcinoma from squamous cell carcinoma (Table 7.3).
Table 7.3
Summary of immunohistochemical stains in the differential diagnosis of poorly differentiated carcinoma of lung
TTF-1 | Napsin A | p63 | |
|---|---|---|---|
Adenocarcinoma | +a | +a | − |
Squamous cell carcinoma | − | − | + |
7.1.6.8 Large Cell Carcinoma
Large cell carcinoma (LCC) is an undifferentiated carcinoma without cytologic and architectural features of small cell carcinoma and glandular or squamous differentiation.
7.1.6.9 Gross Pathology
LCCs usually present as large, peripheral masses, often invade visceral pleura, chest wall, or adjacent structures. Typical cut surface is gray-tan tumor with frequent necrosis and occasional hemorrhage.
7.1.6.10 Histopathology
Characteristic features are sheets or nests of large polygonal cells with vesicular nuclei, prominent nucleoli, and a moderate amount of cytoplasm.
7.1.6.11 Metastatic Tumors to the Lung
Secondary tumors in the lung are more common than primary lung neoplasms. Detecting the organ of origin is frequently difficult, particularly metastatic adenocarcinoma of unknown primary. Multiple-marker panels of immunohistochemical stains are developed to predict the primary site as shown in Table 7.4.
Table 7.4
Immunohistochemical stains for differential diagnosis of metastatic lesion or unknown origin
CK7 | CK20 | TTF-1 | CDX2 | GCDFP-15 | CEA | Mucin | |
|---|---|---|---|---|---|---|---|
Lung | + | − | ± | − | − | − | MUC5AC− |
Breast | + | − | − | − | + or ER+ | − | − |
Colorectum | − | + | − | + | + | MUC2+ | |
− | − | − | + | ||||
Stomach | + | − | − | + | |||
Ovary | + | − | − | − | − | MUC5AC+ | |
Pancreaticobiliary tract | + | − | − | − | + | MUC5AC+ |
7.1.7 Tumor Staging and Staging Workup
One of the most universal lung cancer staging methods utilizes the 7th edition of TNM system developed by the International Association for the Study of Lung Cancer (IASLC) as shown in Tables 7.5 and 7.6 [56], with a large database, the broad international spectrum, careful data analysis, and complete validation [57]. This system was approved by the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) [58]. The nodal status is the best prognosis of tumor recurrence and overall survival, therefore, to achieve most accuracy for nodal status, IASLC defined seven zones as follows: a supraclavicular zone (Station 1), an upper zone (Station 2R-4R, 2L-4L), an aortopulmonary (AP) zone (Station 5 and 6), a subcarinal zone (Station 7), a lower zone (paraesophageal; Station 8 and inferior pulmonary ligament; Station 9), a hilar/interlobar zone (hilar; Station 10 and interlobar; Station 11), and a peripheral zone (Station 12–14) [59] as shown in Fig. 7.1.
Table 7.5
Summary of 7th edition TNM system developed by IASLC
TX | Positive cytology only |
|---|---|
T1 | ≤3 cm |
T1a | ≤2 cm |
T1b | More than 2–3 cm |
T2 | Main bronchus ≥2 cm from carina, invades visceral pleura, partial atelectasis/obstructive pneumonia extending to hilum but not involving the entire lung |
T2a | >3–5 cm |
T2b | >5–7 cm |
T3 | >7 cm; direct invasion to chest wall, diaphragm, pericardium, phrenic nerve, mediastinal pleura, main bronchus <2 cm from carina, total atelectasis or obstructive pneumonia entire lung, separate nodule(s) in same lobe |
T4 | Tumor direct invasion to mediastinum, heart, great vessels, carina, trachea, oesophagus, vertebra, and recurrence laryngeal nerve; separate tumor nodule(s) in a different ipsilateral lobe |
N0 | No nodal metastasis |
N1 | Metastasis to ipsilateral peribronchial, ipsilateral hilar and intrapulmonary lymph nodes, including involvement by tumor direct extension |
N2 | Metastasis to subcarinal, ipsilateral mediastinal lymph nodes |
N3 | Metastasis to contralateral mediastinal or hilar lymph nodes, ipsilateral or contralateral scalene or supraclavicular lymph nodes |
M0 | No distant metastasis |
M1a | Separate tumor nodule(s) in a contralateral lobe; pleural nodules or malignant pleural or pericardial effusion |
M1b | Distant metastasis |
Table 7.6
Stages of disease according to TNM system
Staging | T | N | M |
|---|---|---|---|
Occult carcinoma | TX | N0 | M0 |
Stage 0 | Tis | N0 | M0 |
Stage IA | T1a,b | N0 | M0 |
Stage IB | T2a | N0 | M0 |
Stage IIA | T2b | N0 | M0 |
T1a,b | N1 | M0 | |
T2a | N1 | M0 | |
Stage IIB | T2b | N1 | M0 |
T3 | N0 | M0 | |
Stage IIIA | T1a,b, T2a,b | N2 | M0 |
T3 | N1,N2 | M0 | |
T4 | N0,N1 | M0 | |
Stage IIIB | T4 | N2 | M0 |
Any T | N3 | M0 | |
Stage IV | Any T | Any N | M1 |
Other stage classifications such as the American Joint Committee on Cancer (AJCC) which classified stages into two types having individual T, N, and M descriptors; clinical staging and pathologic staging. Clinical staging (pretreatment staging) refers to any information obtained including history taking, physical examination, imaging, endoscopy, biopsy, and surgical procedures before initiation of definite treatment. Pathologic staging (postsurgical staging) refers to all information obtained those through completion of definitive surgery [60], however, this classification creates confusion because of obvious classifications similar to clinical staging nonetheless yields results that can define a pT or pN descriptor, and the overall classification can be a fusion of both classified stage individual [57]. Recently, The IASLC Lung Cancer staging project are proposed for the revisions of the T descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. T descriptors were classified as follows: T1 was subclassified into three groups, T1a (no more than 1 cm), T1b (>1 to 2 cm), and T1c (>2 to 3 cm). T2 was subclassified into two groups, T2a (>3 to 4 cm) and T2b (>4 to 5 cm). Tumors greater than 5 cm to less than or equal to 7 cm were classified as T3. Tumors greater than 7 cm were classified as T4. Tumors involving main bronchus regardless of distance from carina were classified as T2. Tumors causing partial or total atelectasis/pneumonitis were classified as T2. Tumors invading diaphragm were classified as T4, and mediastinal pelura invasion was deleted from T descriptor [61].
Detterbeck et al. developed new system to classify the completeness of resection known as Residual Tumor Classification as follows: R0 refers to no residual tumor, R1 refers to microscopically positive margin because of positive margin or extracapsular extension at margins of resected nodes or positive pleural or pericardial cytology, and R2 refers to macroscopic residual tumor at the resection margin or resected or unresected nodes or pleural or pericardial nodules [62].
T staging is easy but mediastinal staging (N staging) is more difficult. The methods for mediastinal staging are divided into two techniques; invasive and non-invasive technique. Non-invasive technique should be performed firstly to identify a mediastinal node which can be a guide for invasive technique and then, for tissue diagnosis, the invasive technique should follow. Non-invasive techniques include CT scan; 55 % of sensitivity and 81 % specificity, PET scan; 80 % sensitivity and 88 % specificity, or PET/CT; 62 % of sensitivity and 90 % of specificity. Invasive techniques are subdivided into two methods; surgical methods and needle methods. Surgical methods include mediastinoscopy (approach to mediastinal lymph node station 1, 2R, 2L,4R, 4L, and 7); 81 % sensitivity, VATS approach for station 2–10; 99 % sensitivity, include transthoracic needle aspiration (TTNA); 94 % of sensitivity, transbronchial needle aspiration (TBNA); 78 % of sensitivity, Endoscopic ultrasound-guided needle aspiration (EUS-NA); 89 % of sensitivity, real-time EUS-guided TBNA; 89 % of sensitivity, real-time EBUS-TBNA and EUS-NA; 91 % sensitivity. All of these invasive techniques have 100 % specificity [63]. The chosen technique for tumor staging depends on the location of the tumor, mediastinal lymph node and availability of diagnostic tools.
ACCP guideline 2013 summarized that patients suspected to have lung cancer, a chest CT should be performed and PET scan can be done if available. In clinical Stages III and IV NSCLC, MRI or CT brain should be performed, even if the patient has a negative clinical evaluation. In patients with mediastinal lymph node positive from PET, but negative from chest CT, invasive staging is recommended. In patients with high suspicion of N2,3 involvement, a needle technique should be performed first [63] (Fig. 7.4).
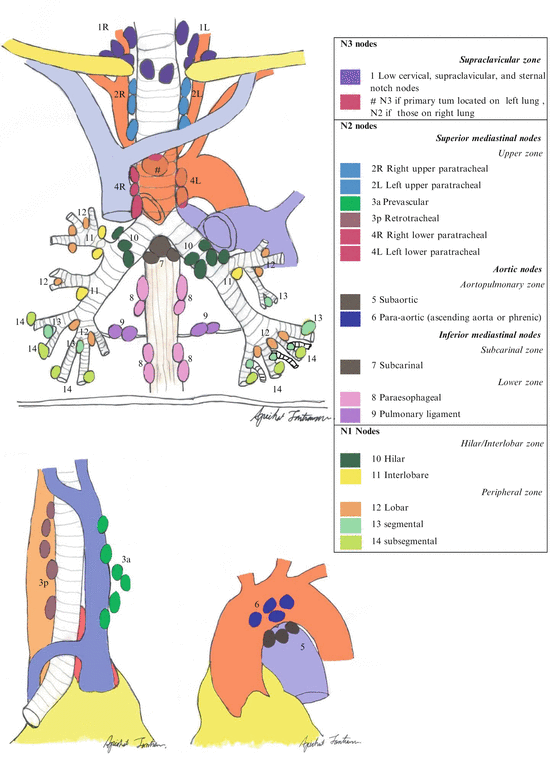

Fig. 7.4
Lymph node mapping according to the International Association for the Study of Lung Cancer (IASLC)
7.1.8 EGFR, KRAS, ALK-EML4 and other molecular aberrations in NSCLC
Epidermal growth factor receptor (EGFR) is expressed on the surface of the cell of NSCLC. EGFR mutation is the best molecular predictor for response in patients receiving treatment with EGFR-tyrosine kinase inhibitor (EGFR-TKI). Prevalence of EGFR mutations in NSCLC of any histology were ranged from 8.4 % to 35.9 % in ever or heavy smokers and from 37.6 % to 62.5 % for never or light smokers. For adenocarcinoma subtype EGFR mutations were more commonly found in Asian (47.9 %) than in Western patients (19.2 %) [64]. The activating of EGFR mutations was more commonly associated with female, Asian ethnicity, and never smoker [65]. The majority of EGFR mutations in tyrosine kinase occur as in-frame deletion in exon 19, exon 21 (L858R) substitution mutation, and exon 18 G719x [66]. Only the mutation in exon 20 T790M is associated with TKI resistance [67].
The prevalence of KRAS mutation is in approximately 25 % of NSCLC patients. They are more common in adenocarcinoma and in smoker patients [68]. They were also associated with poor prognosis and resistance to EGFR-TKI [69].
ALK-EML4 (Anaplastic lymphoma kinase oncogene fusion with other gene such as echinoderm micirotubule-associated protein-like 4) occurs in approximately 2–7 % of NSCLC [70–75, Tantraworasin et al. 2014]. They are more common in adenocarcinoma histology, never or former light smoker and younger patients [71]. The ALK fusion gene tends to be mutually exclusive with EGFR and KRAs mutations. Other rare fusion of ALK with other partners has also been identified [76]. The gold standard method for detection of ALK gene rearrangement is fluorescent in situ hybridization assay.
EGFR, KRAS, EML4-ALK mutations all tend to be exclusive [77]. Other molecular aberrations in NSCLC are MET/hepatocyte growth factor receptor (HGFR), ROS, and RET oncogenes. The summary of molecular aberrations, prevalence, and clinical relevance are shown in Table 7.7.
Table 7.7
Molecular aberrations, prevalence, and clinical relevance in NSCLC
Biomarkers | Prevalence | Genomic aberration | Clinical relevance |
|---|---|---|---|
EGFR | EGFR mutations in non-squamous histology 15 % in Caucasians 40 % in Asians 75–80 % in never-smoker Asians EGFR mutations in squamous histology 5 % EGFR over-expression 39 % in adenocarcinoma 58 % in squamous cell carcinoma 38 % in large-cell carcinoma | Activating mutation within intracellular catalytic domain of EGFR Over-expression of extracellular part of EGFR | Tyrosine kinase inhibitors (e.g., gefitinib, erlotinib, and afatinib or other second or third generation) (Good response in Exon 19 deletions and Exon 21 L858R point mutation) Monoclonal antibodies (e.g., cetuximab and necitumumab) |
ALK | 2–7 % in unselected NSCLC 10 % in non-never-smokers <1 % in squamous carcinoma | Chromosomal translocation and fusion of ALK gene | Tyrosine kinase inhibitors (e.g., crizotinib and ceritinib) |
MET | 2–4 % MET amplification (untreated) 5–20 % MET amplification in EFGR-TKI-resistant tumors 25–75 % over-expression of extracellular part of MET receptor | Increased MET copy number Over-expression of extracellular part of MET receptor | Tyrosine kinase inhibitors (e.g., tivantinib, cabozantinib, and crizotinib) Moniclonal antibodies (onartuzumab, AMG 102, ficlatuzumab) |
ROS-1 | 1–2 % in unselected population | Chromosomal translocation and fusion of ROS-1 gene | Tyrosine kinase inhibitor (crizotinib) |
KRAS | Rare in never-smokers 25–30 % in adenocarcinoma 5 % in squamous cell carcinoma | Activating mutation within catalytic RAS domain | Downstream pathway inhibitors (e.g., MEK inhibitors selumetinib and trametinib) |
7.1.9 Treatment Modalities
A multidisciplinary approach for NSCLC is recommended for achieving intense curative treatment including surgery, chemotherapy, radiotherapy, targeted therapy and immunotherapy. Choosing a treatment modality mainly depends on the stage of disease and patient status.
7.1.9.1 Surgery
(A)
Surgery for early stage NSCLC (Stage I and Stage II)
Surgery is a primary approach for early stage, Stage I and II, NSCLC if there are no contraindications. Anatomical resection such as lobectomy or larger is recommended. Sleeve or bronchoplastic resection is recommended more than a pneumonectomy because of its affect on the quality of life and no greater survival benefit. The 3rd edition of ACCP guideline recommended that surgery should be performed by a board certified thoracic surgeon with a focus on lung cancer. The general thoracic surgical procedures would be performed in more than 75 % of the thoracic surgeon’s clinical practice, and also include an average performance of at least four anatomical resections per month to maintain the experience [79]. Systematic mediastinal lymph node sampling or dissection should be done simultaneously with anatomical resection. There are no statistically significant differences between these two methods in terms of disease-free survival and tumor recurrence after complete resection in Stage I NSCLC patients as proven by the largest randomized control trial study [80]. For clinical Stage II, systematic mediastinal lymph node dissection may provide an additional survival benefit rather than mediastinal lymph node sampling [79]. In the sampling procedure, at least six lymph nodes per station should be sampled for accurate pathologic node staging suggested by AJCC/UICC, however, IASLC recommend three mediastinal node stations (N2 nodes), one of which must be the subcarinal node (station 7), and three N1 nodes/stations should be sampled [79]. Several other guidelines such as the European Society of Thoracic Surgeons (ESTS) guidelines [81], and Cancer Care Ontario (CCO) guidelines [82] have recommended that at least three mediastinal lymph nodes stations, one of which must be Station 7 (subcarinal nodes) and at least ten lymph nodes including both N1 and N2 nodes. Darling et al. summarized in Thoracic Surgical Clinics that at least three N2 group nodes (station 2–9), one of which must be station 7, and removal of 10–16 lymph nodes in total including at least stations 10 and 11 [83].
There are three surgical approaches for lung cancer surgery; conventional open thoracotomy, video-assisted thoracoscopic surgery (VATS) and robotic surgery. A surgeon can perform all approaches utilizing oncologic principles. Nowadays, many studies confirm that the VATS approach is safe, can achieve oncologic principles of lung cancer resection (anatomical resection and mediastinal lymph node dissection or sampling). Moreover, the advantages of the VATS approach are a shorter hospital stay and reaching a 5 year and disease-free survival compared to an open thoracotomy [84–91]. Robotic surgery for lung cancer resection and lymph node dissection is comparable for radicality, safety [92] and 5 year survival (91 % in Stage IA and 88 % in Stage IB) [93] to VATS and open surgery and achieve similar results. Zhang et al. performed systematic review and meta-analysis to compare the outcome of surgery between VATS and open thoracotomy approach and found that there was no significant difference in the number of total lymph node dissection or sampling between the two groups. Systemic (Risk ratio (RR): 0.61; 95 % CI: 0.48–0.78; P < 0.01) and loco-regional (RR: 0.66; 95 % CI: 0.46–0.95; P = 0.03) recurrence rates were significantly lower in the VATS group. Moreover, a significantly higher survival rate (RR: 1.09; 95 % CI: 1.03–1.15; P < 0.01) was also demonstrated by a Forest plot in the VATS group. These results suggest that VATS lobectomy might be an eligible alternative in place of thoracotomy in patients with early-stage NSCLC by reducing recurrence and improving survival rates [94]. Use caution in interpreting these meta-analysis results, the RR of survival rate of VATS is only 1.09 and statistical significance does not mean clinical significance.
In patients with poor pulmonary reserve and defined as having maximal oxygen consumption (VO2 max) less than 10 mL/kg/min, or the combination of a maximum VO2 less than 15 mL/kg/min with both FEV1 and DLCO less than 40 % predicted postoperative (PPO) function, there is an increased risk for postoperative cardiovascular and respiratory complications after lung resection [95]. The ACCP guideline 2013 [79] recommends that sublobar resection (segmentectomy if possible) with 2 cm gross margins can be performed in clinical Stage I NSCLC. For patients where adequate margin could not be achieved, the addition of brachytherapy mesh to a sublobar resection may improve local control. Recent studies [96, 97] found that sublobar resection and lobectomy had equivalent survival for patients with clinical Stage IA, however, the incidence of locoregional recurrence is higher than in lobectomy [98]. Oparka et al. [99] summarized eight studies that compared VATS with conventional techniques for lung resection in patients with poorly reserved lung. They found that a VATS approach has similar perioperative outcomes to those with normal lung function regardless of the type of resection, including lobectomy.
(B)
Surgery for locally advanced stage (Stage III)
The role of surgery in Stage III NSCLC is still debatable especially in N2 disease. Currently, there are two strategies in surgical treatment. The first strategy, suggested by Mehran in 2013 based on best evidence that patients who were proved to be pathologic N2 disease and no evidence of metastasis and presented with bulky multistation of N2, definitive concurrent chemotheraphy and radiation therapy should be first considered, whereas without bulky multistation (only one station bulky of N2) either definitive concurrent chemotherapy and radiation therapy or induction chemotherapy, followed with radical surgical resection (R0) should be considered. In case of persistent disease after surgery, Postoperative radiation therapy with chemotherapy should be performed [100]. The second strategy, suggested by ACCP guideline 2013, this guideline did not suggest to use the term “potentially resectable” or “unresectable” because they are subjective, depend on the individual decision and experience of surgeons, but divide the patients diagnosed as Stage III NSCLC into three subgroups; (1) patients with infiltrative tumor Stage III (N2/N3), defined as tumor infiltrated into mediastinum partially surrounding the vital structures such as great vessels or trachea; (2) patients with occult N2 node involvement despite thorough preoperative staging; and (3) patients with discrete clinically evident N2 involvement by CT scan or CT-PET scan), defined as mediastinal nodes can be separated. Surgery has a role only in later subgroups, however, definitive chemoradiation therapy or induction therapy (either chemotherapy alone or combined with radiation) followed by surgery is recommended over either surgery or radiation alone because it can downstage the tumor [101–105]. Anatomical resection with systematic mediastinal lymph node sampling or complete dissection is recommended [105, 106]. If patients with discrete N2 disease identified preoperatively (IIIA), primary surgical resection followed by adjuvant therapy is not recommended, however, if incidental (occult) N2 disease was found at surgical resection despite fully preoperative staging methods, planning for complete resection with mediastinal lymphadenectomy should be continued because of achieving 87 % of 3-year survival and 81 % of 5-year survival [105, 107]. VATS approach can be safely performed in selected cases [91]. Both of strategies for surgical resection in stage III NSCLC should be performed under a discussion of the multidisciplinary team which include a minimum a thoracic surgeons, medical oncologist, and radiation oncologist. Pneumonectomy should be avoid as much as possible because of high mortality and morbidity, therefore in case of planning for pneumonectomy after induction therapy, patients should be advised of increased operative risk, the postoperative mortality was 21 % (odds ratio = 4.01; p = 0.0007) and a predictor of postoperative mortality was a postoperative bronchopleural fistula [108].
(C)
Surgery for Stage IV
Actually, treatment for Stage IV NSCLC is multimodality treatment, including chemotherapy, radiotherapy, targeted therapy and immunotherapy. Surgery may be a role in some circumstances especially patients suffered from its complication such as massive hemoptysis or obstructive pneumonitis, however, risk and benefit should be considered especially in case of T4 which tumor invade vital structures such as the heart [109], main trunk of pulmonary artery or main bronchus. Surgical treatment in synchronous brain metastasis has been interesting issue since 1988 [110]. An absence of mediastinal node metastasis is a favorable prognostic factor In the past many studies reported the benefit of these strategies [111–116]. Recent study demonstrated that an overall survival rates of bifocal surgical resection of synchronous brain metastasis and primary NSCLC were 79 %, 42 %, and 8 % at the 1st, 2nd, and 5th years, respectively and median survival was found to be statistically significantly lower for the stage T3 tumors when compared with both stage T1 and T2 tumors (p = 0.037), furthermore, the most benefit from surgery will occur when no mediastinal lymph node involvement or any other extrathoracic spread [117]. Gamma-knife radiosurgery can be used effectively and beneficially instead of conventional brain surgery [118–121]. General indication for using gamma-knife radiosurgery for brain metastasis in lung cancer include; (1) Karnofsky Performance Scale (KPS) ≥70; (2) estimated life expectancy ≥ 4 months; (3) no rapidly evolving intracranial mass effect; (4) three or fewer lesions with maximum diameter ≤ 3 cm; (5) target (s) well defined on the neuroimages; (6) stage I or II of NSCLC; and (7) no extracranial metastasis [122, 123].
7.1.9.2 Radiosurgery
Stereotactic single-dose radiotherapy, using dose ranged between 19 and 30 Gy/isocenter, is safe and effective treatment option for early stage NSCLC patients who were not suitable for surgery even in minimally invasive surgery [124], however it was associated with significant local progression [125]. Overall survival rates and disease-free survival rates at 12 and 36 months were 74.5 %, 37.4 %, and 70.2 %, 49.1 % respectively. The local tumor control rates at 12 and 36 months were 89.5 % and 67.9 % respectively [126]. In 2009, Ahn et al. suggested that CyberKnife treatment was very safe and able to achieve a high local control rate, suggesting for alternative therapeutic modality in early lung cancer [127]. A current retrospective cohort study demonstrated that this method had similar survival, locoregional control and total recurrence control to surgery after controlling for prognostic and patient selection factors. However, randomized clinical trials are needed to answer which one is better focusing on effectiveness of treatments [128]. Not only in early stage NSCLC, the role of this method in advanced stage also be evaluated in combined with gefitinib as a second-line or third-line treatment in patients with advanced NSCLC [129]. Most patients tolerated it well with Grade 1–2 side effects and no Grade 4 or higher toxicity was identified. The clinical disease-related symptom improvement rate was 57.1 % with median duration 8.0 months of symptom improvement. The 1 year local control and OS rates were 83.9 % and 69.6 %, respectively. The median progression-free survival and OS were 7.0 and 19.0 months, respectively. They summarized that radiosurgery combined with gefitinib was a promising treatment strategy for advanced (Stage IIIb or IV) NSCLC after the failure of previously chemotherapy. Local control and disease-related symptoms were improved with tolerated toxicity, and even increased the progression-free survival and OS.
7.1.9.3 Chemotherapy, Radiotherapy, Targeted Therapy and Immunotherapy
(A) Early Stage
Adjuvant Therapy
Locoregional recurrence after completely resection of tumor is common in approximately 20–25 % in Stage I–II and up to 50 % in Stage III, adjuvant platinum-based chemotherapy has become standard in patients with Stage II and IIIA NSCLC [130]. From several randomized, controlled trials and meta-analyses adjuvant chemotherapy provides a significant survival advantage with 5 year absolute benefit approximately 5 % [131–133]. Cisplatin in combination with vinorelbine appears to be preferable to other combination regimens. The LACE, ANITA and JBR 10 trials reported that cisplatin combination with vinorelbine had the greater effect on overall survival when compared with other drugs [132, 134–136]. If surgical margin is positive or presence of pN2 disease, postoperative radiation is considered. Modern techniques of radiation are recommend to reduce toxicity and improve outcome [130, 137, 138].
(B) Locally Advanced Stage
Sequential or Concurrent Chemotherapy and Radiation
In patients with unresectable locally advanced or medically inoperable Stage III NSCLC and good performance status, a concurrent chemoradiation with platinum-based chemotherapy is preferred to sequential chemotherapy and radiation. Median survival was 14.6 months for sequential therapy versus 17 month s in concurrent therapy [139]. Data from meta-analysis identified a significant benefit of concurrent chemoradiation on overall survival (HR 0.84) and 5 year absolute benefit of 4.5 % but there were significant esophageal side effects [140]. For definitive radiation, standard dose RT (60 Gy) is commonly used and overall survival is similar to high dose radiation (74 Gy) [141, 142].
Neoadjuvant Chemotherapy Followed by Surgery
The results from meta-analysis showed that a neoadjuvant chemotherapy arm improved in overall survival superior to surgery alone arm [143, 144]. The delivery of chemotherapy is more difficult in the postoperative setting (adjuvant therapy) when compared with preoperative chemotherapy as demonstrated in NATCH phase III trial [145]. However, neoadjuvant chemotherapy had similar benefit to postoperative chemotherapy [132, 143].
Radiotherapy
Radiotherapy alone is considered in patients who are not fit for chemotherapy or with poor performance status.
(C) Advanced Stage
Chemotherapy
Meta-analyses have proved that platinum-based chemotherapy improves overall survival when compared with best supportive care and gain median survival time from 4.5 to 6 months and increased 1 year survival from 20 % to 29 % [146]. Doublet combination of second generation chemotherapy with platinum-based regimen for four to six cycles is the standard of care in advanced NSCLC. The second generation drugs such as docetaxel, gemcitabine, paclitaxel and vinorelbine are used in combination with platinum [130]. Randomized clinical trial showed similar outcomes in term of response rate, progression free survival and overall survival of second generation chemotherapy either paclitaxel or gemcitabine or docetaxel in combination with platinum [147]. Phase II trial demonstrated that non-platinum based chemotherapy had inferior progression free survival to platinum-based regimen. However, phase 3 trial data show no statistically difference in median survival between platinum or nonplatinum doublet chemotherapy [130]. From meta-analysis trial showed carboplatin had similar overall survival when compared to cisplatin and appears less toxic, especially nausea, vomiting and nephrotoxicity [148]. In patients with non-squamous NSCLC (adenocarcinoma and large cell) pemetrexed/cisplatin had a statistically significant better survival than gemcitabine/cisplatin [149]. However, patients with squamous cell lung cancer the pemetrexed/cisplatin regmin had inferior survival to gemcitabine/cisplatin. In patients with performance status at least two are usually treated with single agent chemotherapy includes gemcitabine, pemetrexed, taxanes or vinorelbine. Combination chemotherapy regimens include paclitaxel/carboplatin, pemetrexed/carboplatin from randomized control trial had significantly improve survival survival when compare with single agent pemetrexed alone with median OS was P = 5.3 months vs. CP = 9.3 months (HR = 0.62, 95 % CI 0.46; 0.83, p = 0.001) [150]. However some patients had treatment-related deaths.
7.1.9.4 Molecular Therapy (Targeted Therapy)
First Line Setting
EGFR: Targeted Agents
A large randomized study (IPASS) compared EGFR- tyrosine kinase inhibitor (gefitinib) with standard chemotherapy (paclitaxel/carboplatin) in first line setting of light or never smoked, Stage IIIB or IV adenocarcinoma of lung. Progression free survival (PFS) was significantly better with gefitinib in EGFR mutation group, however overall survival is not difference between gefitinib and standard chemotherapy. The most common adverse events in the gefitinib group were rash or acne (66.2 %) and diarrhea (46.6 %). whereas neutropenia, neurotoxicity (69.9 %), neutropeia (67.1 %) and alopecia (58.4 %) in paclitaxel/carboplatin arm [151]. Interstitial pneumonitis is the uncommon serious adverse event of EGFR-TKI that should be monitored in addition to progression of disease or other causes. The randomized Phase 3 study evaluated EGFR-TKI (erlotinib) versus standard chemotherapy in adenocarcinoma of lung stage IIIB/IV harbouring activating EGFR mutation. The result showed a significant improve PFS in patients received erlotinib and better tolerability when compared to chemotherapy arm [152–154].
Afatinib is an irreversible ErbB family blocker and was studied compared to chemotherapy (pemetrexed/cisplatin) in patients with adenocarcinoma of lung whose tumors harboured EGFR mutation. The results from the LUX-Lung 3 trial showed that afatinib group had prolongation of PFS with median PFS of 11.1 months versus 6.9 months in the chemotherapy arm (HR, 0.47; 95 % CI, 0.34–0.65; P = 0.001. The most common adverse events of afatinib were diarrhea, rash/acne, and stomatitis/mucositis [155].
ALK-Targeted Agent
Crizotinib, an ALK inhibitor, has been shown to be effective against ALK positive NSCLC. From Phase II study (PROFILE 2005) in second and third line treatment showed dramatic responses of 60 % with a median PFS of 8.1 months. It was generally well tolerated and low toxicity. The common adverse events were edema, dizziness, nausea, decreased appetite, diarrhea, constipation, visual effects, increased liver transaminases and fatigue. It is also c-MET inhibitor and ROS1 inhibitor. Crizotinib was granted for ALK-positive NSCLC based on clinical efficacy and safety data from Phase I and Phase II trial [156]. All patients with non-squamous cell NSCLC should be testing for the presence of EGFR mutation and ALK rearrangement and EGFR-TKI or ALK inhibitor should be used as first-line therapy in patients with known EGFR mutation or ALK rearrangement. Randomized study show that targeted therapy improved progression free survival when compared with standard chemotherapy and have fewer adverse events even overall survival is not different [152–154].
Anti-EGFR Antibody
A monoclonal antibody (Cetuximab) targeting the epidermal growth factor receptor (EGFR) was assessed in advanced NSCLC patients in randomized phase III trial (FLEX). The data demonstrated that the addition of cetuximab to standard chemotherapy (cisplatin/vinorelbine) prolonged overall survival for a median of 11 months compared with 10 months for chemotherapy alone. However, the benefit was slightly improved survival and it was not clinically significant [157].
Antiangiogenesis Agents
Bevacizumab is a monoclonal antibody against vascular endothelial growth factor. Bevacizumab combined with paclitaxel based regimen is another choice for patients with non-squamous advanced NSCLC based on the results from phase II trial (ECOG 4599) with statistically improved overall survival. The median survival was 12.3 months in bevacizumab combination with chemotherapy group versus 10.3 months in chemotherapy without bevacizumab group. Meta-analysis showed that bevacizumab prolongs the progression free survival and overall survival when added to doublet platinum-based chemotherapy with HR 0.72 and 0.90 respectively [158]. Overall survival benefit was found only in combination of bevacizumab and paclitaxel/carboplatin and should not be used in squamous cell carcinoma and recent history of hemoptysis. Other anti-angiogenic agents such as Vandetanib, a small molecule inhibitor of VEGF signaling, EGFR and RET or sorafenib showed no benefit in overall survival [159].
Maintenance Therapy
The goal of treatment in advanced stage is to improve symptom and maximize overall survival time. An optimal duration of chemotherapy in the first line is usually four to six cycles due to minimize potential toxicity. Maintenance therapy with the cytotoxic agents and targeted drugs that can prolong progression free survival, overall survival, not detrimental to quality of life and patients can tolerated for prolonged period, and cost not expensive should be the important properties of acceptable drugs used in maintenance phase. Maintenance therapy has two approaches [160], switch maintenance and continuous maintenance therapy. First, switch maintenance is the transition from standard platinum-based chemotherapy to different chemotherapy or targeted therapy. Second, continuous maintenance is to continue non-platinum chemotherapy of the initial platinum-based regimen. From randomized controlled trials, switch maintenance therapy with pemetrexed [161] or erlotinib [162] or continuation maintenance with bevacizumab [163], cetuximab [157], pemetrexed [164], had significantly improvement in progression free survival and overall survival when given in patients who did not progress after four cycles of platinum-based chemotherapy. For erlotinib maintenance [162], the overall benefit was significantly better in patients with stable disease after first line chemotherapy, but not in responder patients. Erlotinib had greater benefit in patients with EGFR mutations. Other drugs such as switch maintenance with docetaxel [165] or continuation maintenance with gemcitabine [166] or bevacizumab/pemetrexed [167] had been tested and results showed improve progression free survival but not for overall survival. Maintenance treatment in patients with NSCLC is not a standard of care for all patients; it is only an option in some patients. The implementation of maintenance therapy remains debated regarding the switch or continuation of maintenance, type of agents, and optimal duration. There are many questions of maintenance in clinical practice and clinical trials [160] including: (1) four cycles of platinum-based chemotherapy seem to be insufficient for survival benefit, (2) whether the control arm in the clinical trials received the appropriate second line treatment, (3) what is the best endpoint for the maintenance trial, (4) the optimal time between early and late introduction of subsequent treatment and others. Well designed and randomized controlled trials in this area are warranted.
Second Line and Third Line Systemic Treatment
Platinum based chemotherapy with or without bevacizumab is a choice for second line therapy after failure from first line targeted agents (EGFR-TKI or ALK inhibitor). Second or third line treatment, both docetaxel and pemetrexed (only for nonsquamous cell carcinoma) are recommended in patients who had progression of disease, if chemotherapy had never been given and with performance status of zero to two. Randomized studies demonstrated the overall survival and quality of life improvement with docetaxel compares with ifosfamide, vinorelbine or best supportive care [168, 169]. Pemetrexed showed less toxicity, similar in response rate, progression free survival, and overall survival [170]. A meta-analysis study compared single agent with combination chemotherapy in second line treatment. Results showed that combination chemotherapy had significantly improved response rates and progression-free survival, but not improve overall survival and increased toxicity [171]. Regarding targeted agents, BR.21 trial test between erlotinib (EGFR-TKI) versus best supportive care in second or third line treatment, the overall survival is better in the erlotinib arm with median overall survival 6.7 months versus 4.7 months in best supportive arm [172]. Gefitinib (EGFR-TKI) demonstrated noninferior overall survival when compares with docetaxel [173]. Crizotinib, an ALK-inhibitor had efficacy in second or third line setting NSCLC after previous chemotherapy who had ALK rearrangement. The overall response rate and stable disease are 57 % and 33 % respectively. The 1 and 5 year overall survivals are 74 % and 54 % respectively [71]. Targeted agents include EGFR-TKI (gefitinib, erlotinib), ALK-inhibitor (crizotinib) can be given in patients with a performance of three to four because these agents had lower hematologic side effects with tolerability.
7.1.9.5 Immunotherapy
An immunotherapy approach for lung cancer is an attractive concept. It has the potential to improve the outcome of treatment, immune-progression-free survival and overall survival based on nonrandomized and randomized Phase II and Phase III trials. Large randomized Phase III trials are currently in process. The anti-EGF vaccine has been evaluated in randomized Phase IIB study with stage IIIB/IV NSCLC patients who completed first-line chemotherapy. The treatment group trended toward improved survival when compared with the control group [174, 175]. The Mycobacterium vaccae (SRL172) a promoter of autologous antigen recognition was conducted to study randomized Phase III with advanced stage NSCLC to receive vaccine administered concurrently with chemotherapy for six cycles followed by maintenance or control group. However there was a high dropout rate which limited the statistical power. The results showed that the vaccine group had a significantly improved quality of life without affecting overall survival in all patients. Survival benefit was found in patients with adenocarcinoma who completed the vaccine schedule when compared with control group or patients with squamous cell carcinoma [176, 177]. GVAX, an autologous tumor cell vaccine, was evaluated in nonrandomized in patients with early and advanced NSCLC. Three of 33 patients with advanced stage achieved complete response and prolonged remission. Eight of ten patients with early stage had disease free survival more than 12 months [178]. An allogenic antigen approach (Lucanix) was evaluated as a phase II nonrandomized trial with early and late stage NSCLC. Results showed that Lucanix had 15 % response and increased survival when compared with the historical control patients [179]. BLP25 liposome vaccine (Stimuvax) which is immune adjuvant between mucin-1 protein with monophosphoryl lipid A. The randomized phase II trial showed no statistical difference in overall survival but trended to improve median survival in subgroup of patients with stage IIIB locoregional disease when compared to the control group (30.6 versus 13.3 months) [180]. MAGE-A3 Antigen-Specific Cancer Immunotherapy was studied in randomized Phase IIB trial and showed non-statistical significance delayed time to recurrence (35.0 % in vaccine group versus 43.0 % in control group) [181]. This interesting result introduced MAGE-A3 for the investigation of the efficacy in preventing cancer relapse in large randomized Phase III trial (MAGRIT). TG410 vaccine is a recombinant virus expressing MUC1 antigen and interlukin-2. It was tested in Phase II study and showed enhancement of the effect of chemotherapy by a improved response rate and trended to improve progression free survival [182]. Ipilimumab is a fully human monoclonal antibody that stimulates immunity by anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4). From phase II study, Ipilimumab when used concurrent or phased ipilimumab combined with chemotherapy showed improved median immune-related progression-free survival. It was 5.68 months for the phased ipilimumab group versus 4.63 month for chemotherapy alone group (HR 0.68, p = 0.02) and 5.52 months for concurrent ipilimumab group versus 4.63 for chemotherapy alone group (HR = 0.77, p = 0.09). The important adverse events were hypophysitis, enterocolitis and hyperthyroidism which may be improved with steroids. The Phase III trials are still ongoing [183].
7.1.9.6 Radiation for Palliative Treatment
Palliative radiotherapy is an important option for patients with symptomatic metastatic stage or locally advanced stage not suitable for curative treatment. Radiotherapy has demonstrated the benefit to improve respiratory problems such as hemoptysis, dyspnea, tracheal or bronchial compression and chest pain. Palliative radiotherapy also plays role in painful bone metastases, symptomatic brain metastases and superior vena cava syndrome [130, 184]. High dose rate brachytherapy provided better symptomatic palliative treatment especially in patients with endobronchial lesion rather than external beam radiation alone [185].
7.1.10 Conclusion
The incidence of lung cancer continues to increase but its mortality has plateaued or slightly decreased which may be due to improvement in multidisciplinary treatment. Low-dose CT screening is a very interesting issue for early detection of lung cancer and has reduced the overall mortality. Further studies should be continued for the evaluation of cost-effectiveness. Staging workup techniques are very important for definite diagnosis and planning of treatment. Multi-modality treatment including surgery, radiosurgery, radiotherapy, chemotherapy, targeted therapy and immunotherapy should be considered in all stages of NSCLC. The aims of treatment are for cure, especially in early stages, or at least to improve the quality of life in advanced disease.
7.2 Small Cell Lung Cancer
7.2.1 Incidence
The incidence of small cell lung cancer has decreased to approximately 12.95 % in newly diagnosed lung cancers. This could be explained by the decrease in prevalence of smokers because smoking remains the predominant risk factor for this disease [186].
7.2.2 Pathology
The histology of SCLC is a poorly differentiated epithelial tumor of small cells with scant cytoplasm. SCLC is currently designated as high-grade neuroendocrine carcinoma (neuroendocrine carcinoma, grade 3) together with large cell neuroendocrine carcinoma (Table 7.8), thus grading is inappropriate.
Table 7.8
Systems of nomenclature for neuroendocrine tumors
Grade | WHO [54] | Moran et al. [187] |
|---|---|---|
Low grade | Carcinoid tumor | Neuroendocrine carcinoma, Grade 1 |
Intermediate grade | Atypical carcinoid tumor | Neuroendocrine carcinoma, Grade 2 |
High grade | Small cell carcinoma | Neuroendocrine carcinoma, Grade 3, small cell carcinoma |
Large cell neuroendocrine carcinoma | Neuroendocrine carcinoma, Grade 3, large cell neuroendocrine carcinoma |
7.2.2.1 Gross Pathology
SCLCs are usually white-tan, soft, friable perihilar tumors with massive necrosis and often nodal metastasis. They typically spread along bronchi in a submucosal and circumferential fashion with frequently extensive lymphatic invasion.
7.2.2.2 Histopathology
The tumors exhibit a wide spectrum of architectures including nest, trabeculae, strands, and rosette formation. Single cell fashion or sheet-like growths without typical neuroendocrine morphology are also common as shown in Fig. 7.5. SCLC cells usually have round, ovoid or spindled nuclei and scant cytoplasm. Characteristic cytologic features include ill-defined cytoplasmic borders, finely granular nuclear chromatin, absent or inconspicuous nucleoli, and prominent nuclear molding. Mitotic rate is high. The diagnosis can be confirmed by using the panel of IHC including chromogranin A, synaptophysin, and CD56.
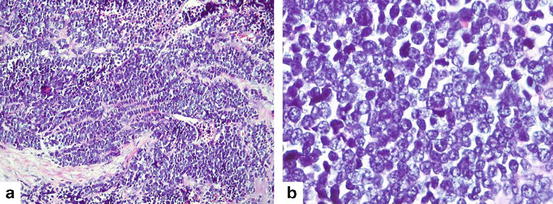

Fig. 7.5
Small cell carcinoma (a) Solid sheets and occasional trabeculae of densely packed malignant cells showing scant cytoplasm, finely granular chromatin (Hematoxylin and eosin 200×). (b) Neoplastic cells show round nuclei with finely granular chromatin, absence of nucleoli and scant cytoplasm. High mitotic rate is typical feature (Hematoxylin and eosin 400×)
7.2.2.3 Clinical Presentation
Small-cell lung cancer (SCLC) is characterized by more aggressive behavior and early development of widespread metastases. The proportion of new cases in limited stage small cell lung cancer is approximately 40 %. When compared with NSCLC, SCLC is more responsive to chemotherapy and radiation initially but relapse occurs quickly, with a 5 year survival rate of less than 10 % [186]. Brain metastases are common in SCLC. At the time of diagnosis, approximately 10–14 % of patients with SCLC will have brain metastases [189].
Paraneoplastic syndromes such as Cushing syndrome, carcinoid syndrome, Lambert-Eaton myasthenia syndrome, dermatomyositis, thrombocytosis or thromboembolism are more commonly presentations clinically in SLCL than those in NSCLC, especially in Cushing syndrome (up to 50 % of SCLCs) or SIADH (Syndrome of Inappropriate Antidiuretic Hormone, up to 45 %) [18]. Other clinical presentations in NSCLC also can present in SCLC such as chronic cough, hemoptysis, or chest pain. Because SCLC is usually located at the central part of the respiratory airway, superior vena cava syndrome is also more common than in NSCLC.
7.2.3 Investigation and Staging Workup
The investigations and staging workup for SCLC include, history taking, physical examination, chest CT, PET or PET/CT, MRI, bone scan, bone marrow aspiration or biopsy. The aim of treatment in limited-disease is curative intent, therefore, metastasis must be identified by routine procedures.
The role of PET or PET/CT scan for initial staging of SCLC has been evaluated in many studies. In summary, it can provide 16 % up-stage disease and also 11 % of down-stage disease, compared with conventional imaging, which influence the decision making process, approximately 30 % change in treatment [190]. Moreover, current studies found that patients with limited-stage evaluated by PET achieved an improved disease control and survival comparing with non-PET scan. The overall survival was 32 months in PET-staged patients and 17 months in non-PET-staged patients (p = 0.03). The better intrathoracic disease evaluation may explain these findings [191]. Therefore, in patients with clinically limited-stage SCLC, PET scan is suggested [190].
SCLC staging is classified into two stages; limited stage and extensive stage according to TNM staging [56]. Limited stage includes T any, N any, M0, that be safe for definite radiotherapy, except T3-T4 due to multiple lung nodules or lesion and lymph nodes that are too large that do not tolerate definite radiotherapy. Extensive stage includes T any, N any, M1a/1b or T3-T4 due to multiple lung nodules.
7.2.4 Treatment Modalities
Treatment modalities of SCLC include chemotherapy, radiotherapy, radiosurgery and surgery. Chemotherapy and radiotherapy have a primary role, however, for curative-intent, especially in limited disease; surgery or radiosurgery should be considered.
7.2.4.1 Surgery
Radiotherapy and chemotherapy are primary treatments of SCLC, however, the surgical role has been intensively studied since 1966 [192]. A large population database, US population-based database from 1988 to 2002 with 14,179 SCLC patients and 863 (6.1 %) of these who underwent surgery were analyzed. Surgical was more commonly performed in limited disease and had longer survival than in the non-surgical group. Patients with localized disease underwent lobectomy had a median survival of 65 months and a 5-year OS of 52.6 % whereas patients who had regional disease had a median survival of 25 months and a 5-year OS rate of 31.8 %. Only N 2 disease patients received a benefit from adjuvant radiotherapy [193]. Another larger database, The National Cancer Institute Surveillance Epidemiology and End Results (SEER) database from 1988 to 2004 with 1,560 stage I SCLC patients was analyzed to evaluate outcomes between surgical and non-surgical groups. They found that the 5 year survival in patients who underwent lobectomy with postoperative radiotherapy was comparable with those without postoperative radiotherapy (50 % versus 57 %, respectively) [194]. The ACCP guideline 2013 and NCCN guideline 2014 summarized that surgical resection is recommended in patients with clinical stage I (T1-T2,N0) SCLC after being fully evaluated in distant metastasis and invasive mediastinal staging (head MRI/CT and PET or abdominal CT plus bone scan) and these patients should receive platinum-based adjuvant chemotherapy if pathologic nodal negative, and concurrent chemotherapy with mediastinal radiotherapy [190], 195
7.2.4.2 Chemo: Radiotherapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree