On CT, normal lung varies in appearance, depending on the window settings used. With a window mean of –600 to –700 Hounsfield units (HU) and a width of 1000 to 1500 HU, the lungs appear dark, but not as black as the air visible in the trachea or bronchi. This slight difference in attenuation between lung parenchyma and air should be sought in choosing an appropriate window setting. If the lungs are viewed with too high a window mean, soft-tissue structures in the lung (vessels, bronchi, or lung nodules) are difficult to see or are underestimated in terms of their size, and any areas of lucency, such as bullae, may be missed. If too low a window mean is used, the size of soft-tissue structures in the lung will be overestimated.
The following discussion of lung diseases is divided into six parts, each part reviewing an important topic in the CT diagnosis of lung disease. Separate reading lists are provided for each part.
A: Lobar Anatomy, Lobar Pneumonia, and Atelectasis
The Fissures and Lobar Anatomy
The lobes are delineated by the major and minor fissures ( Fig. 6.1 ).

Major Fissures
The major fissures separate the lower lobes posteriorly from the upper lobe on the left and the middle and upper lobes on the right. They are not clearly visible on CT with 5-mm collimation but are easily seen as thin white lines on CT with thin slices ( Fig. 6.1 ). The lung parenchyma immediately adjacent to the fissures, being peripheral, contains few visible vessels and appears relatively avascular. The major fissures are incomplete in many patients (i.e., they do not completely separate the lobes).
Within the lower thorax, the major fissures angle anterolaterally from the mediastinum, contacting the anterior third of the hemidiaphragms. In the upper thorax, the major fissures angle posterolaterally. Above the aortic arch, they contact the posterior chest wall.
Minor Fissure
The minor fissure separates the right middle lobe from the right upper lobe. Although it parallels the scan plane, and is more difficult to see than the major fissure, it is usually visible on thin slices as a white line of varying sharpness and thickness. The relatively avascular lung on both sides of the fissure is visible on CT in most patients. In some patients the minor fissure mimics the appearance of the major fissure but is seen anterior to it.
Because the minor fissure often angles caudally, the right lower, middle, and upper lobes may all be seen on a single scan. If the minor fissure is concave caudally, it can sometimes be seen in two locations or can appear ring-shaped ( Fig. 6.2 ), with the middle lobe between the fissure lines or in the center of the ring and the upper lobe anterior to the most anterior part of the fissure.

Accessory Fissures
In patients with an azygos lobe, the four layers of the mesoazygos, or azygos fissure, are invariably visible above the level of the intrapulmonary azygos vein. The azygos fissure is C-shaped and convex laterally, beginning anteriorly at the right brachiocephalic vein and ending posteriorly at the right anterolateral surface of the vertebral body ( Fig. 3.21 ). Other accessory fissures, most commonly the inferior accessory fissure (separating the medial basal segment of either lower lobe from the other lower lobe segments), are occasionally seen on CT. They are not generally of diagnostic significance.
Lobar Pneumonia
Pneumonia usually results in lung consolidation. The term consolidation is used to describe the presence of homogeneous lung opacity resulting from replacement of alveolar air by something else, with obscuration of the underlying vessels and bronchial walls; air bronchograms (visible air-filled bronchi) are typically visible but not invariably present. On enhanced CT, vessels with a normal appearance and course can often be seen within consolidated lung. In contrast, a mass or space-occupying lung lesion usually displaces vessels or bronchi, or they may be narrowed or invisible because of compression or invasion.
In some patients with pneumonia, consolidation involves most of a lobe or an entire lobe; this is termed lobar pneumonia . In a patient with lobar pneumonia, the volume of the consolidated lobe is normal or sometimes increased ( Fig. 6.3 ). Usually the infectious organisms responsible for lobar pneumonia lead to an exuberant watery exudate. The exudate easily spreads from one alveolus to another, resulting in an expanding sphere of consolidation that stops when it reaches a fissure. If lobar pneumonia is imaged early in its evolution, it may appear as a rounded, poorly marginated area of consolidation. This is sometimes referred to as round pneumonia. Common causes of lobar pneumonia include Streptococcus pneumoniae , Klebsiella pneumoniae , Legionella , Mycoplasma , Mycobacterium tuberculosis , and some viruses and fungi. In addition to pneumonia, other causes of lobar consolidation include obstructive pneumonia (lung consolidation associated with a central bronchial obstruction, even if uninfected), invasive mucinous adenocarcinoma, aspiration, pulmonary edema, and in rare cases, pulmonary hemorrhage or a vascular abnormality (e.g., pulmonary embolism).

Pulmonary infections can also present with other CT patterns. These are discussed later in this chapter and include patchy consolidation ( bronchopneumonia or lobular pneumonia ); single or multiple nodules, masses, or cavities; tree-in-bud opacities; centrilobular nodules; ground-glass opacity (GGO); and miliary or random nodules.
Atelectasis: Types and Patterns of Lobar Collapse
Atelectasis most commonly occurs because of bronchial obstruction ( obstructive atelectasis ), pleural effusion or other pleural processes that allow the lung to collapse ( passive or relaxation atelectasis ), or lung fibrosis ( cicatrization atelectasis ). These conditions have different appearances. Generally, the signs of volume loss on CT are the same as those on chest radiographs. Mediastinal shift (particularly of the anterior mediastinum), elevation of the diaphragm, and displacement of fissures are well seen on CT.
Obstructive Atelectasis
Obstructive atelectasis often occurs because of lung cancer or other bronchial tumors, and the airways should be examined closely. Lobar atelectasis commonly results; typically, the affected lobe is partially or completely consolidated ( Fig. 6.4 ). Air bronchograms are usually, but not always, absent. Mucus bronchograms (low-density fluid or mucus within obstructed bronchi) can sometimes be seen on CT. The air- or mucus-filled bronchi can be dilated in the presence of atelectasis, simulating bronchiectasis. If contrast medium infusion is used, opacified vessels are often visible within the consolidated and collapsed lobe (a collapsed lung is not simply airless; the alveoli are usually filled with fluid). If little volume loss is present, the term obstructive pneumonia is often used ( Fig. 6.5 ).


On CT, atelectasis can be diagnosed when displacement of fissures is seen. Typical patterns of lobar collapse can be identified ( Figs. 6.6 and 6.7 ).


Right Upper Lobe Collapse
With collapse the major fissure rotates anteriorly and medially as the upper lobe progressively flattens against the mediastinum ( Figs. 6.4 and 6.6 ). The fissure can be bowed anteriorly. In the presence of a hilar mass, an appearance similar to Golden’s S sign , as seen on plain radiographs, is visible. In some patients the lobe assumes a triangular shape ( Fig. 6.4 ).
Left Upper Lobe Collapse
As on the right, the major fissure rotates anteromedially. However, above the hilum the superior segment of the lower lobe may displace part of the upper lobe away from the mediastinum, giving the posterior margin of the collapsed lobe a V shape ( Fig. 6.6 ). A similar appearance is sometimes seen on the right ( Fig. 6.4 ). On chest radiographs this appearance results in the so-called Luftsichel sign .
Middle Lobe Collapse
As the middle lobe loses volume, the minor fissure rotates downward and medially. The collapsed lobe assumes a triangular shape, with one side of the triangle abutting the mediastinum ( Fig. 6.7 ). The upper lobe can be seen anterolaterally, bordering the collapsed lobe, with the lower lobe bordering it posterolaterally. These aerated lobes usually separate the collapsed middle lobe from the lateral chest wall.
Lower Lobe Collapse
On either side the major fissure rotates posteromedially ( Fig. 6.7 ). The collapsed lobe contacts the posterior mediastinum and posteromedial chest wall and maintains contact with the medial diaphragm.
Passive Atelectasis
In the presence of pleural effusion the lung tends to retract or collapse toward the hilum, and fluid entering the fissures allows the lobes to separate. With injection of contrast medium, the collapsed lung opacifies and is clearly distinguishable from surrounding fluid. Air bronchograms may be seen within collapsed lobes. Rounded atelectasis is a form of passive atelectasis. Linear, disk, or platelike atelectasis at the lung bases may be seen as a result of limited diaphragmatic motion.
Rounded Atelectasis
Rounded atelectasis represents focal, collapsed, and often folded lung. It is common and almost always occurs in association with pleural thickening or pleural effusion.
Rounded atelectasis is most common in the posterior paravertebral regions and may be bilateral in patients with bilateral pleural abnormalities. Areas of rounded atelectasis, which appear as a mass or mass-like consolidation, are usually several centimeters in diameter. Bending or bowing of adjacent bronchi and arteries toward the edge of the area of rounded atelectasis, because of volume loss or folding of lung, is characteristic ( Fig. 6.8 ); this finding has been termed the comet-tail sign . Air bronchograms can sometimes be seen within the mass. Rounded atelectasis opacifies after contrast medium infusion.

Four findings must be present to make a confident diagnosis of rounded atelectasis on CT; if these are present, follow-up is usually unnecessary ( Fig. 6.8 ). However, if one or more of these findings is lacking, you should be cautious in making the diagnosis, and close follow-up or biopsy may be necessary. The four findings of rounded atelectasis are:
- •
ipsilateral pleural effusion or thickening
- •
significant contact between the lung lesion and the abnormal pleural surface
- •
the comet-tail sign
- •
volume loss in the lobe in which the opacity is seen
Rounded atelectasis may be associated with asbestos-related pleural thickening, occurring adjacent to regions of thickened pleura, but often has an atypical appearance. Areas of atelectasis or focal fibrosis in patients exposed to asbestos can be irregular, may not have extensive pleural contact, and may not be associated with the comet-tail sign.
Cicatrization Atelectasis
Cicatrization atelectasis occurs in the presence of pulmonary fibrosis and may be associated with tuberculosis (TB), radiation, or chronic bronchiectasis. In this condition, there is no evidence of bronchial obstruction. Rather, air bronchograms and bronchial dilatation (bronchiectasis) are usually visible within the area of collapse. The volume loss is often severe.
Suggested Reading
B: Congenital Lesions
Congenital lung lesions may result from anomalous development of bronchi, lung parenchyma, vasculature, or a combination of these. The most common or significant abnormalities are reviewed.
Pulmonary Agenesis and Aplasia
Pulmonary agenesis consists of the complete absence of a lung and its bronchi and vascular supply. With pulmonary aplasia, a rudimentary bronchus is present, ending in a blind pouch, but lung parenchyma and pulmonary vessels are absent ( Fig. 6.9 ).

Tracheal Bronchus
The origin of all or part (usually the apical segment) of the right upper lobe bronchus from the trachea is termed a tracheal bronchus ( Fig. 6.10 ); its incidence is less than 1%. A left-sided tracheal bronchus supplying part of the left upper lobe is much less common. Tracheal bronchus is common in cloven-hoofed animals such as pigs, sheep, goats, camels, and giraffes; it is sometimes called a pig bronchus or bronchus suis . It may be associated with recurrent infection.

Bronchial Atresia and Congenital Lobar Overinflation
Bronchial atresia is characterized by local narrowing or obliteration of a lobar, segmental, or subsegmental bronchus. It is most common in the left upper followed by the right upper and middle lobes. Mucus commonly accumulates in dilated bronchi distal to the obstruction, resulting in a tubular, branching, or ovoid mucus plug or mucocele . The lung remains aerated distal to the obstruction because of collateral ventilation, but air trapping occurs, and the obstructed lobe or segments often appear hyperlucent and hypovascular. Bronchial atresia is usually diagnosed in adults. Many patients are asymptomatic although infection of obstructed lung may occur.
Congenital lobar overinflation (CLO), formerly known as congenital lobar emphysema , is characterized by marked overinflation of a lobe. Most cases present within the first month of life; symptoms of respiratory distress are typical. Most cases of CLO are associated with partial or complete bronchial obstruction occurring as a result of deficient cartilage, external compression, or luminal obstruction. CLO is most common in the left upper lobe (40%), middle lobe (35%), and right upper lobe (20%) (note the similarity of this distribution to that of bronchial atresia). Mediastinal shift away from the abnormal lobe usually occurs. Resection is often necessary. It is reasonable to assume that cases of CLO that go unrecognized at birth may be diagnosed years later as bronchial atresia.
Bronchogenic Cyst
The appearance of a mediastinal bronchogenic cyst is described in Chapter 4 . Pulmonary bronchogenic cysts are typically well defined, round or oval, and of fluid or soft-tissue attenuation; previously infected cysts can contain air or an air-fluid level. When a cyst contains air, its wall appears very thin, although consolidation of surrounding lung may be present. Such cysts are most common in the lower lobes.
Arteriovenous Malformation
Pulmonary Arteriovenous Malformation
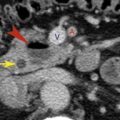
Pulmonary arteriovenous malformation (AVM) is supplied by the pulmonary artery(s) and drained by the pulmonary vein(s). Pulmonary AVM can be single (65%) or multiple (35%) and is often associated with Osler-Weber-Rendu syndrome (65%). On CT, pulmonary AVM can appear as a single dilated vascular sac or fistula, visible as a smooth, sharply defined round or oval nodule (most common), or a tangle of dilated tortuous vessels, appearing as a lobulated or serpiginous mass. In each type the feeding pulmonary artery (or arteries) and the draining pulmonary vein (or veins) are dilated and should be easily seen on CT ( Fig. 6.11 ).

An AVM with a single feeding artery and draining vein ( simple AVM ) is most common; a complex AVM with multiple supplying vessels is less frequent. In most cases the fistula is immediately subpleural in location. These characteristic findings are generally sufficient to make a specific diagnosis on scans without contrast medium infusion, even for AVMs only a few millimeters in diameter.
Although contrast medium is not usually needed for diagnosis, a pulmonary AVM shows rapid and dense opacification after bolus contrast medium injection, followed by rapid washout of the contrast ( Fig. 6.11 ). As would be expected, opacification occurs just after opacification of the right ventricle and pulmonary artery. For a fistula larger than 3 mm, catheter embolization is the treatment of choice. Catheter pulmonary angiography is generally performed in association with embolization.
Systemic Arteriovenous Malformation
Rarely, an anomalous systemic artery arising from the aorta or one of its branches supplies an area of otherwise normal lung. Drainage into pulmonary veins is via normal capillaries rather than through a dilated vascular sac or fistula. Systemic AVM is usually diagnosed incidentally, although hemoptysis may occur. In contrast to sequestration, described next, systemic AVM supplies normally aerated lung, containing normal bronchi (i.e., there is no sequestered lung).
Pulmonary Sequestration
Pulmonary sequestration represents a focal lung anomaly, without normal bronchial or pulmonary artery supply, fed by an anomalous systemic artery or arteries. Between 70% and 90% of sequestrations are located posteriorly and medially at the left lung base. In most cases the feeding systemic artery(s) is visible on contrast-enhanced CT ( Figs. 6.12 and 6.13 ). There are two types.


Intralobar Sequestration
Intralobar sequestration is most common and is usually diagnosed in adults. It is contained within an otherwise normal lobe, usually the left lower lobe. Recurrent or chronic infection is common. Venous drainage is usually by means of pulmonary veins, although systemic (azygos) vein drainage can be seen. Intralobar sequestration typically contains air but can be quite variable in appearance. On CT, intralobar sequestration can appear as
- •
a region of hyperlucent lung ( Fig. 6.12A )
- •
a cystic or multicystic abnormality (sometimes with air-fluid levels)
- •
consolidated or collapsed lung ( Fig. 6.13 )
- •
a combination of these findings
Areas of lucent lung in association with or representing part of an intralobar sequestration are common ( Fig. 6.12A ). Normal bronchi are not seen in the sequestration, and if the sequestration is aerated, the vascular branching pattern within it may appear abnormal.
Extralobar Sequestration
Extralobar sequestration is relatively uncommon and is usually diagnosed in infants or children. Infection is rare. It has its own pleural envelope, rarely contains air, and almost always appears as a solid mass. Venous drainage is usually by systemic veins.
Congenital Pulmonary Airway Malformation
Congenital pulmonary airway malformation (CPAM), also known as congenital cystic adenomatoid malformation , consists of an intralobar mass of disorganized lung tissue, derived primarily from bronchioles, and often cystic or multicystic. About 70% present during the first week of life, but 10% are diagnosed after the first year, and some are first diagnosed in adults. Lower lobes are most often involved, but any lobe can be affected.
Five types (numbered 0–4) of CPAM have been described, on the basis on their histologic features. In adults, CPAM usually presents as an air-filled, or air- and fluid-filled cystic or multicystic mass. It may also appear as a solid mass or focal consolidation. It may mimic sequestration on CT but is less common.
Hypogenetic Lung (Scimitar) Syndrome
Hypogenetic lung syndrome (also known as scimitar syndrome and venolobar syndrome ) is a rare anomaly almost always occurring on the right side. The term scimitar syndrome derives from the scimitar-like appearance of the anomalous vein that is commonly present. It is characterized by four features that coexist in various degrees:
- •
hypoplasia of the lung with abnormal segmental or lobar anatomy;
- •
hypoplasia of the ipsilateral pulmonary artery;
- •
anomalous pulmonary venous return (the scimitar vein ) from the right upper lobe or the entire right lung, usually to the vena cava or right atrium;
- •
anomalous systemic arterial supply to a portion of the hypoplastic lung, usually the lower lobe.
On CT the hypoplastic right lung is recognizable because of dextroposition of the heart and mediastinal shift to the right ( Fig. 6.14 ). The hypoplastic lung may also show abnormal bronchial anatomy, deficient bronchial divisions, or mirror-image bronchial or pulmonary artery branching. When an anomalous (scimitar) vein is present, it is clearly visible on CT, often draining into the right atrium or inferior vena cava. Hypoplasia of the pulmonary artery is usually recognizable by the decreased size of vessels in the hypoplastic lung. This entity may be associated with congenital heart disease.

Suggested Reading
C: Solitary Pulmonary Nodule and Focal Lung Lesions
CT is often used to evaluate a solitary nodule, mass, or focal lung lesion. It is used to (1) evaluate morphology , which can sometimes be diagnostic (e.g., pulmonary AVM), (2) determine attenuation , including the presence of ground-glass opacity (GGO), soft-tissue attenuation, calcium, fat, or opacification after contrast medium infusion, (3) assess growth rate (or absence of growth) for nonspecific lesions when a more aggressive approach is not warranted, and (4 ) plan procedures , such as percutaneous biopsy or resection or other interventions. CT is also used to detect lung nodules in patients being screened for lung cancer or being followed up for a known malignancy, and in conjunction with positron emission tomography (PET) scanning (i.e., PET-CT) to evaluate the metabolic activity of a nodule or lung lesion.
To make a semantic point, the term nodule is usually used to refer to a focal lung lesion 3 cm or less in diameter, which is relatively well defined, roughly spherical, and at least partially outlined by lung. Mass is used for a lesion larger than 3 cm. These measurements also distinguish a T1 tumor (≤3 cm) from a T2 to T4 tumor (>3 cm) in the lung cancer staging system ( Table 4.3 ).
With multidetector CT, a 1- to 1.5-mm slice thickness is optimal, but lung cancer screening may be done with thicker slices (i.e., 2–5 mm) and a low-dose technique. A high-resolution reconstruction algorithm should generally be used for image reconstruction.
Morphology of Focal Lesions and Lung Nodules
The differential diagnosis of a solitary nodule or mass is long ( Table 6.1 ). However, lung cancers and some other focal lesions can have characteristic appearances on CT, which may be diagnostic or suggest a limited differential diagnosis.
| Congenital lesions and normal variants |
| Arteriovenous malformation a |
| Bronchogenic cyst |
| Mucoid impaction (bronchial atresia) |
| Sequestration |
| Malignant neoplasm |
| Carcinoma a |
| Lymphoma a |
| Lymphoproliferative disease a |
| Metastatic neoplasm a |
| Lung sarcoma (e.g., chondrosarcoma, liposarcoma, fibrosarcoma) |
| Benign neoplasms and neoplasm-like conditions |
| Hamartoma |
| Lymphoproliferative disease a |
| Benign tumors (e.g., chondroma, lipoma, fibroma) |
| Infection and parasites |
| Angioinvasive aspergillosis a |
| Dirofilaria immitis (dog heartworm) |
| Echinococcus a |
| Focal (round) pneumonia a |
| Granulomatous infection or granuloma (tuberculosis, nontuberculous mycobacteria, fungus) a |
| Lung abscess a |
| Mycetoma (aspergilloma) a |
| Inflammatory (noninfectious) conditions |
| Organizing pneumonia a |
| Rheumatoid nodule a |
| Sarcoidosis a |
| Granulomatosis with polyangiitis (Wegener granulomatosis) a |
| Airway and inhalational disease |
| Mucoid impaction (mucous plug) in bronchiectasis a |
| Conglomerate mass or progressive massive fibrosis (e.g., silicosis) a |
| Lipoid pneumonia a |
| Vascular abnormalities |
| Hematoma |
| Infarction a |
| Septic embolism a |
| Miscellaneous |
| Amyloidosis a |
| Rounded atelectasis |
Lung Cancer
Adenocarcinoma is the most common cell type of lung cancer (50% of cancers) and is the most common cell type presenting as a lung nodule (60%–70%), but any lung cancer cell type can present in this manner. On CT, lung cancers may appear solid, of GGO, or of mixed GGO and solid.
Although a definite diagnosis of lung cancer cannot be made on CT, findings that suggest malignancy in a patient with a solitary nodule include:
- •
an irregular or spiculated nodule margin, usually caused by fibrosis or invasion of surrounding lung (90% of nodules with a spiculated edge are malignant; Fig. 6.15 );

FIG. 6.15
Spiculated invasive adenocarcinoma.
High-resolution CT in a patient with a nodule in the left lower lobe shows a lobulated and spiculated mass typical of carcinoma. Linear opacities contacting the pleural surface (yellow arrows) are termed pleural tails. The nodule contains several air bronchograms or air-filled cystic areas (blue arrows) .
- •
a lobulated contour ( Fig. 6.16 );

FIG. 6.16
Spiculated invasive adenocarcinoma with an irregular cavity.
Six contiguous 1-mm scans through a speculated nodule with a lobulated contour. The nodule contains a thick-walled cavity. Pleural tails are also visible.
- •
air bronchograms ( Fig. 6.15 ) or cystic or “bubbly” air–containing regions within the nodule (seen in 65% of cancers but only 5% of benign lesions);
- •
a nodule exceeding 6 mm that is of GGO or of mixed GGO and solid attenuation (two-thirds are malignant; Fig. 6.17 );

FIG. 6.17
Two adenocarcinomas associated with ground-glass opacity.
(A) A nodule (arrows) consisting of pure ground-glass opacity represents an adenocarcinoma in situ. (B) A nodule with a dense center (arrow) surrounded by a halo of ground-glass opacity (the halo sign) represents a lepidic predominant adenocarcinoma. The soft-tissue attenuation center measures more than 5 mm.
- •
cavitation ( Fig. 6.16 ) with a nodular cavity wall or a wall exceeding 15 mm in greatest thickness (90% are cancers);
- •
an upper lobe nodule;
- •
a diameter exceeding 2 cm (95% are cancers);
- •
associated emphysema or lung fibrosis.
A spiculated edge and the presence of air bronchograms ( Fig 6.15 ) or cystic air-containing regions are particularly common with adenocarcinoma. Lobulation of the nodule also suggests the diagnosis of carcinoma but may also be seen with other lesions, particularly hamartoma.
Both primary lung carcinomas and metastases can cavitate. Typically, a cavitary carcinoma has a thick, irregular, and nodular wall ( Fig. 6.16 ), but some metastatic tumors, particularly sarcomas and those of squamous cell origin, can be thin walled. A cavitary nodule with a thin wall (<5 mm) is most likely (90%) benign.
Adenocarcinoma
Adenocarcinoma most commonly appears as a solid (soft-tissue attenuation) nodule. However, many adenocarcinomas present as a nodule of GGO or a nodule with both GGO and solid components ( Fig. 6.17 ). The term ground-glass opacity (GGO) is used to describe hazy increased lung attenuation that does not obscure underlying vessels or bronchial walls.
The classification of pulmonary adenocarcinomas was revised in 2011 and is summarized in Table 6.2 . Subtypes of adenocarcinoma include adenocarcinoma in situ ( AIS ), minimally invasive adenocarcinoma ( MIA ), and lepidic predominant adenocarcinoma ( LPA ) .
| Premalignant |
| Atypical adenomatous hyperplasia |
| Preinvasive |
| Adenocarcinoma in situ |
| Minimally invasive adenocarcinoma |
| Invasive adenocarcinoma |
| Lepidic predominant adenocarcinoma |
| Invasive adenocarcinoma (various cell types) |
| Variants of invasive adenocarcinoma |
| Invasive mucinous adenocarcinoma |
| Various cell types |
AIS, MIA, and LPA are characterized, at least in part, by lepidic growth , which is defined as tumor growth along alveolar walls without invasion being present, appearing on CT as GGO. Pathologically, AIS is characterized by pure lepidic growth, MIA has lepidic growth with 5 mm or less of invasion, and LPA shows lepidic growth with more than 5 mm of invasion.
Typically, each of the newly described histologic subtypes of adenocarcinoma show some GGO on CT. AIS usually appears as a pure GGO nodule ( Fig. 6.17A ). MIA and LPA usually present as a nodule of mixed GGO and solid attenuation (e.g., the halo sign; Fig. 6.17B ). The term halo sign is used to describe a nodule with a soft-tissue attenuation center surrounded by a less dense halo of GGO. These three types of adenocarcinoma have a relatively good prognosis (100% 5-year survival rate for AIS and MIA, and 75% for LPA) compared with other cell types of lung cancer. A premalignant lesion, atypical adenomatous hyperplasia , typically presents as a GGO nodule 5 mm or less in diameter.
So-called invasive adenocarcinoma has several histologic subtypes. These appear as solid nodules on CT ( Figs. 6.15 and 6.16 ) or a predominantly solid nodule with a small GGO component. Overall, the 5-year survival rate for invasive adenocarcinoma is about 50%. CT findings associated with invasive adenocarcinoma may include spiculation, lobulated borders, air bronchograms, and cavitation. Invasive mucinous adenocarcinoma usually presents with multiple nodules or multifocal GGO or consolidation; it is described later in this chapter and has a poor prognosis.
Although pulmonary adenocarcinoma typically presents with a solitary nodule, up to 20% of patients with adenocarcinoma have multiple synchronous cancers. These may be solid or of GGO.
Other Cell Types of Lung Cancer
Other common cell types of lung cancer include squamous cell carcinoma (15%–20% of lung cancer cases), large cell carcinoma, and small cell (neuroendocrine) carcinoma.
Large cell carcinoma typically appears as a lung nodule or mass, larger on average than adenocarcinoma, but otherwise having a similar appearance.
Squamous cell carcinoma and small cell carcinoma may present with a solid lung nodule resembling invasive adenocarcinoma, but more typically present with central or hilar masses resulting in bronchial obstruction. Small cell carcinoma has a very poor prognosis compared with other cell types of lung cancer.
Other cell types include carcinoid tumor , described in Chapter 5 , and large cell neuroendocrine carcinoma , which resembles large cell carcinoma in appearance.
CT Diagnosis of Nodule Calcification
CT is important in the diagnosis of nodule calcification, a finding that may be very helpful in determining that the nodule is benign. About 25% to 35% of benign nodules appearing uncalcified on chest radiographs show calcification on thin-slice CT. CT with thin slices is optimum for detecting calcium, but dense calcification can also be seen with 5-mm slices. Soft-tissue window settings are best for showing that calcium is present.
When you are using CT to diagnose “benign” calcification, you must be sure that the calcification is benign in appearance ( Fig. 6.18A ). To be reasonably sure of a benign diagnosis, one of the following patterns must be present:
- •
diffuse calcification ( Fig. 6.18B ), typical of a granuloma;
- •
dense, central (i.e., bull’s-eye ) calcification, most typical of histoplasmosis ( Fig. 6.18C );
- •
central and popcorn calcification, typical of hamartoma ( Fig. 6.20B );
- •
concentric rings of calcification ( target calcification; Fig. 6.18D ), typical of histoplasmosis.

A caveat is that sarcomas and carcinoid tumors may be associated with dense calcification that is either homogeneous or “chunky.”
About 5% to 10% of carcinomas contain some calcium, either as a result of tumor calcification or because the carcinoma has engulfed a preexisting granuloma. Calcification in malignancies is typically punctate or stippled or is eccentric within the nodule ( Fig. 6.19 ). Although these patterns of calcification may also be seen in benign lesions, they should be considered to be indeterminate or potentially associated with malignancy.

Hamartoma
CT can be valuable in diagnosing pulmonary hamartoma. Hamartoma usually appears as a smooth and sharply marginated nodule, rounded, or with a lobulated contour. By CT with thin slices, about two-thirds of hamartomas can be correctly diagnosed because of visible fat (60%; Fig. 6.20A ) that is either focal or diffuse, fat and calcification (30%), or calcification (10%). Fat is easily seen on thin-slice CT, with CT numbers ranging between –40 and –120 HU. Calcification may have a “popcorn” appearance because of calcification of nodules of cartilage ( Fig. 6.20B ) or may be diffuse.

Granulomas
Granulomas usually appear rounded and well defined. They may contain calcium, which may be dense and central, diffuse, or on occasion, ring-shaped ( Fig. 6.18 ). An infection or noninfectious granulomatous lesion (e.g., sarcoidosis) presenting as a lung nodule or mass may be associated with small nodules adjacent to or surrounding it (i.e., satellite nodules ; Fig. 6.21 ). In patients with sarcoidosis, this appearance has been referred to as the galaxy sign. Satellite nodules may also be seen with lung carcinoma, but they are evident in only a small percentage of cases.

Tuberculosis, Atypical Mycobacterial, and Fungal Infection
In most patients with TB exposure and a positive PPD skin test result, CT findings are normal. A small lung nodule, calcified or uncalcified and representing the original site of infection, is sometimes visible; associated calcified lymph nodes may be present. Small scattered nodules in the lung apex reflect the presence of hematogenous spread at the time of the original infection. In most patients, these heal without progression.
In a small percentage of individuals, usually children and immunosuppressed patients, primary exposure to TB results in a focal pneumonia (which may involve any lobe). In half of these cases, associated hilar or mediastinal node enlargement is present. Nodes often appear to be low in attenuation on enhanced CT.
Progression of primary TB (often in patients with immune compromise) or after primary reactivation of an earlier infection (usually in the lung apices) can result in cavitation ( Fig. 6.22 ). Cavities are usually irregular in shape and have irregular walls and multiple septations. Air-fluid levels are typically absent, although hemorrhage or superinfection may result in this finding. Cavities may be multiple and associated with satellite nodules.

The spread of infected material through the airways can result in findings of bronchopneumonia (i.e., patchy consolidation, tree-in-bud or centrilobular nodules ), often in regions near the cavity or in the lower lobes ( Fig. 6.22 ). Tree-in-bud and centrilobular nodules are described in the part of this chapter devoted to high-resolution CT (HRCT).
Infection with pathogenic atypical mycobacteria can result in identical findings. Fungal infections can result in findings indistinguishable from TB. If TB is considered in the differential diagnosis, it is generally a good idea to consider fungal infection as well.
The Halo Sign and Angioinvasive Aspergillosis
The halo sign, visible on CT, was first described in immunosuppressed patients with angioinvasive aspergillosis ( Fig. 6.23 ). As indicated in the previous discussion, the term halo sign is used to describe a nodule with a soft-tissue attenuation center surrounded by a less dense halo of GGO.

In patients who are severely immunocompromised, particularly those with treated leukemia and neutropenia, this appearance is typical and highly suggestive of angioinvasive aspergillosis; often treatment is begun without confirmation of the diagnosis. In patients with angioinvasive aspergillosis, the halo sign reflects the presence of a septic infarction (the dense center) surrounded by hemorrhage (the halo).
Although suggestive of angioinvasive aspergillosis in the proper clinical setting, the halo sign is nonspecific and can be seen with other infections (e.g., TB, Legionella infection, nocardiosis, and cytomegalovirus infection), with some tumors (particularly adenocarcinomas, lymphoma, and Kaposi sarcoma), and in patients with pulmonary infarction and granulomatosis with polyangiitis (GPA), formerly termed Wegener granulomatosis . The histologic correlate with the halo varies with the entity. It may represent hemorrhage (e.g., aspergillosis, infarction, Kaposi sarcoma, and GPA), heterogenous consolidation in pneumonia, or lepidic growth of tumor (adenocarcinoma).
The Air-Crescent Sign and Mycetoma
The presence of a lung mass capped by a crescent of air is termed the air-crescent sign (Figs. 6.24 and 6.25 ). It usually indicates the presence of a mass within a cavity. The air-crescent sign is most typical of mycetoma, but it may also be seen in the later stages of angioinvasive aspergillosis, some bacterial infections with lung infarction, cavitary tumors, a clot or neoplasm within a cavity, and echinococcal cysts.


In patients with a preexisting pulmonary cyst or cavity, a mycetoma or fungus ball can form as a result of saprophytic infection, usually by Aspergillus ( Fig. 6.25 ). On CT a round or oval mass (the fungus ball) can be seen in a dependent location within the cavity and is typically mobile. The mass is capped by a crescent of air. Thickening of the cavity wall is common. In patients with a developing mycetoma, the fungus ball can contain multiple air collections. The same appearance can be seen in semiinvasive (chronic necrotizing) aspergillosis, in which the fungus also invades the wall of the cyst or cavity. Hemorrhage and hemoptysis are common associations. With angioinvasive aspergillosis, septic infarction of the lung can result in an air-crescent sign as the patient recovers ( Fig. 6.24 ).
Lung Abscess and Cavities
A lung abscess can occur with a variety of bacterial, fungal, and parasitic infections. The hallmark of a lung abscess is necrosis or cavitation within an area of pneumonia or dense consolidation; the necrotic region can appear quite irregular. Necrosis is commonly visible on contrast-enhanced CT as one or more areas of low attenuation within opacified lung. Cavitation is said to be present if air is visible within the lesion, and CT is often performed to confirm this diagnosis when a plain radiograph is suggestive. In an acute abscess the cavity wall may be irregular or thin and of uniform thickness; with healing the cavity wall is typically thin and uniform. An air-fluid level (or levels) is commonly present with bacterial infection ( Fig. 6.26 ) and is uncommon with cavitary carcinoma, TB, or fungal infection. CT can also be helpful in distinguishing a lung abscess from empyema. Chapter 7 contrasts the CT appearances of lung abscess and empyema.

Pulmonary Infarction and Septic Embolism
Bland infarction with pulmonary embolism can result in a focal pulmonary opacity. Septic emboli are usually multiple. In either instance, opacities typically (1) are peripheral or abut a pleural surface, (2) are round or wedge-shaped, and (3) have a pulmonary artery branch leading to them (the feeding vessel sign ). Some are associated with a halo sign because of surrounding hemorrhage. In patients with septic embolism, cavitation of lung nodules is common. Using contrast-enhanced CT, associated clots may be identified in the proximal pulmonary artery in patients with pulmonary infarction, but a visible clot is not typical of septic embolism.
Lipoid Pneumonia
Chronic aspiration of lipid (animal, vegetable, or mineral) can lead to lipoid pneumonia, with fat and variable amounts of fibrosis resulting in focal consolidations or masses. In some patients, most typically those with mineral oil aspiration, CT shows low-attenuation (–50 to –140 HU) consolidation indicative of its lipid content. When fibrosis predominates, the masses are of soft-tissue attenuation. Lipoid pneumonia differs from hamartoma, which may also contain fat, in that masses are larger and less well defined and usually appear more irregular.
Pleural (Fissural) Lesions
Small (a few millimeters) perifissural nodules are commonly seen on CT and are generally of no concern. Often they are flat or triangular. However, small rounded nodules with minimal fissural contact may represent a significant abnormality, and follow-up may be appropriate, on the basis of its size and suspicious morphology.
Occasionally, a pleural abnormality located in a fissure (e.g., a plaque, loculated effusion, or localized fibrous tumor of the pleura) may be misinterpreted as a lung nodule. Looking for the fissures on the CT will sometimes avoid this mistake.
Nodule Growth Rate and CT Follow-Up of Lung Nodules
The growth rate of a nodule, measured as the time required for doubling of volume (i.e., doubling time , corresponding to a 26% increase in nodule diameter), may be used to determine its likelihood of being malignant. A pulmonary nodule that doubles in volume in less than 1 month or more than 16 months is usually benign. Cancers appearing solid on CT usually have doubling times of 100 to 400 days. However, malignancy should be suspected if any growth occurs, no matter how slow. Carcinomas appearing on CT as a nodule of GGO or mixed GGO and soft tissue have slower growth rates, with doubling times usually ranging from 3 to 5 years.
Small, nonspecific pulmonary nodules are commonly seen on CT performed for other reasons (i.e., an incidental lung nodule). CT is commonly used to follow a newly detected lung nodule or nodules to determine the presence or absence of growth when the nodule size or appearance or clinical factors do not indicate that a more aggressive approach is needed. It is generally agreed that a solid pulmonary nodule that does not grow over a 2-year period is very likely benign and does not require resection.
In a follow-up CT study to assess a small lung nodule or nodules for change in size, it is important to obtain scans with the same slice thickness and view them with the same window settings as in the original study. With spiral CT and volumetric imaging, nodules are not usually missed, but this is possible if the patient breathes during the study. When one is looking for or comparing nodule size, it is important to look at exactly the same levels on the two scans. Identifying and matching the pattern of branching vessels in the peripheral lung in the region of the nodule is the only way of knowing exactly what level is being viewed. If the same vessels are visible on both the original sans and the follow-up scans and the nodule looks different, then the nodule is different. If a different branching pattern is visible, then an apparent difference in nodule size may not be real.
Fleischner Society Guidelines
The Fleischner Society has published recommendations for the follow-up of incidental lung nodules detected with CT (i.e., lung nodules detected in patients not being screened for lung cancer or being followed up for a known cancer) that were widely accepted. The Fleischner Society has recently revised its recommendations for incidental lung nodules appearing solid ( Table 6.3 ) or subsolid (entirely of GGO, or partly of GGO and partly solid; Table 6.4 ). Its guidelines are tailored to provide criteria that will result in follow-up of nodules with a greater than 1% chance of malignancy (approximately). It is important to emphasize that these recommendations do not apply to patients younger than 35 years, in whom lung cancer is rare, immunosuppressed patients, patients with a known cancer, or patients undergoing CT for lung cancer screening. Recommendations for follow-up and management of patients undergoing CT screening (Lung-RADS) are described later.