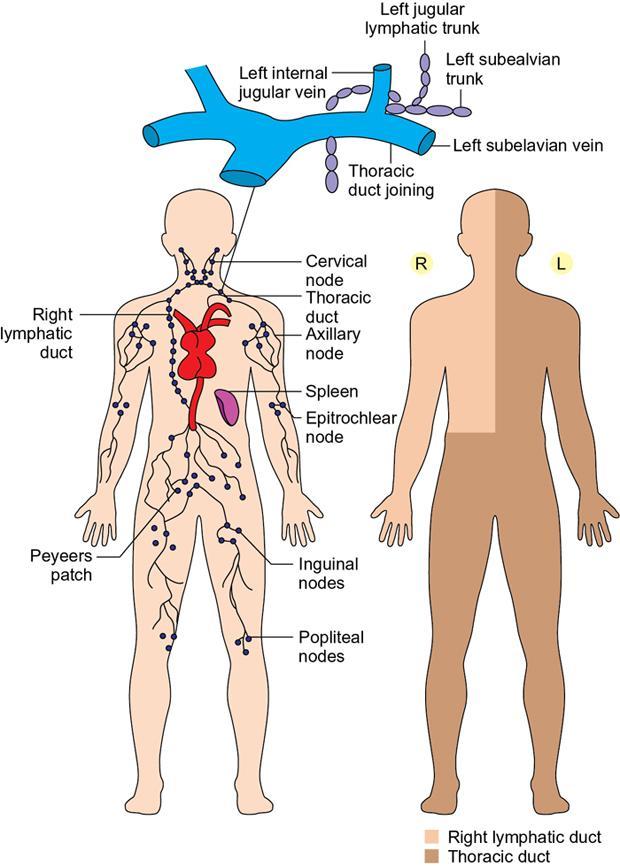
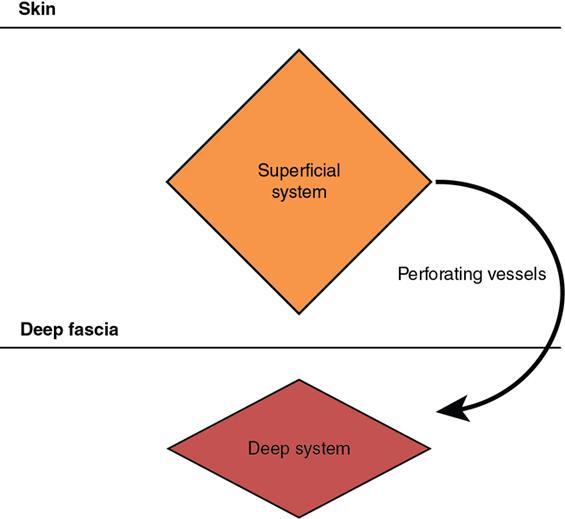
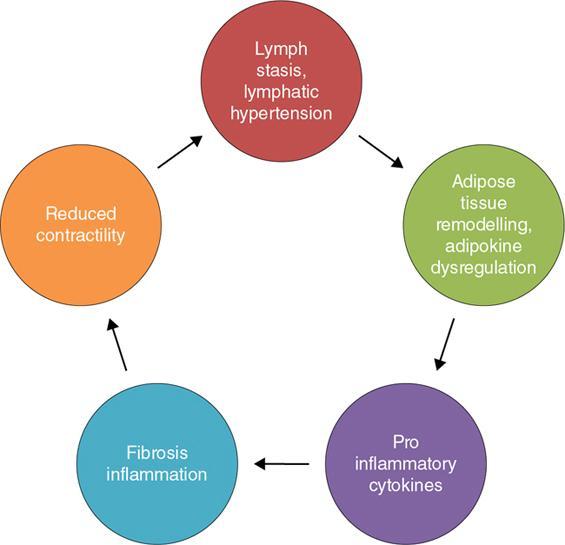
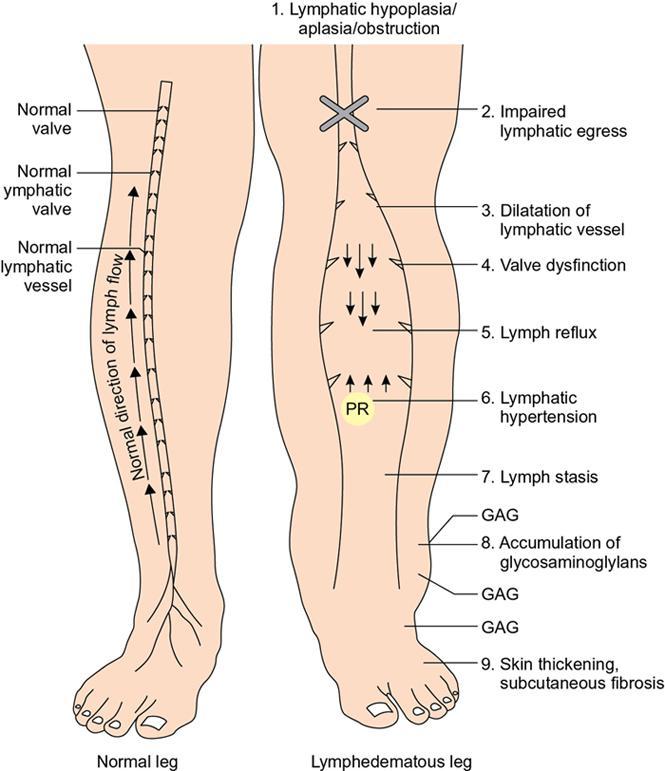
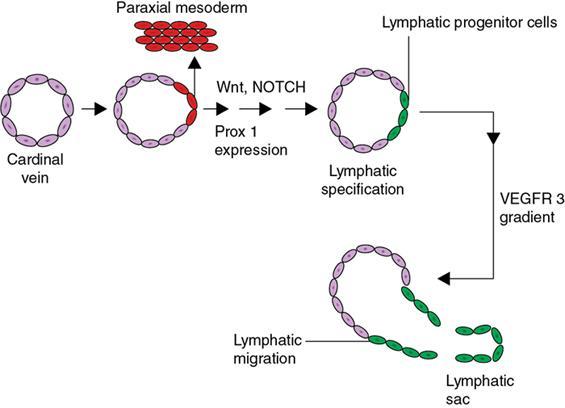
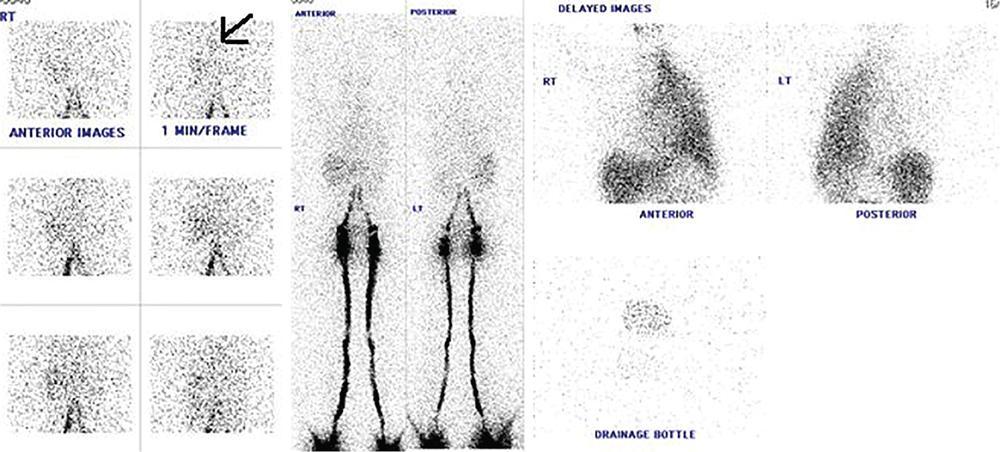
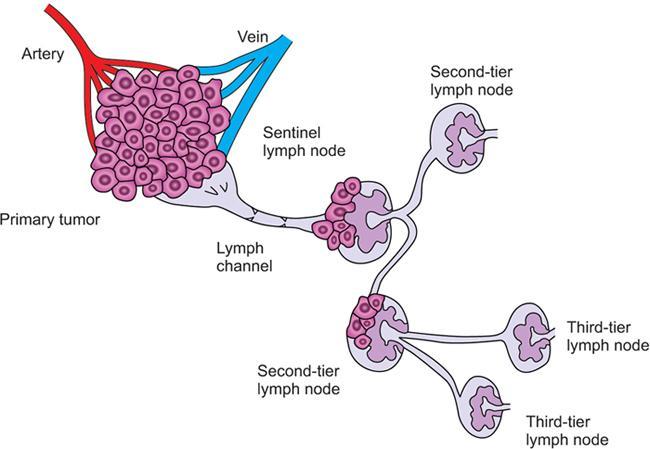
Indirani M, Shelley Simon, Shema Mathew Lymphedema (LED) is a common phenomenon affecting 150–300 million worldwide. It is a prevalent disease which carries a very low mortality but affects the quality of life in a large way. If diagnosed early, the morbidity can be reduced and quality of life can be improved significantly. However, the diagnosis of LED is a great challenge to the clinicians, especially in its early stages. Over the time, from the era of limb measurements to diagnose LED, the diagnosis and management have seen a shift in paradigm with the advent of radionuclide lymphoscintigraphy and MR lymphangiography. LED is due to lymphatic system insufficiency and inadequate lymph transport, leading to pathologic accumulation of protein-rich fluid in the interstitium with resultant inflammation, adipose tissue hypertrophy and fibrosis. It most commonly involves the upper and lower extremities, but can also occur in the face, neck, chest wall, abdomen and genitals. The condition may be associated with disabling local sequelae or life-threatening systemic syndromes. It is the earliest imaging technique achieved to visualize the lymphatic system. An intradermal injection of patent blue dye is used for outlining the superficial lymphatic vessels. Then vessel is cannulated with a specially designed 30-gauge needle to infuse an oil-soluble iodinated contrast medium. Due to challenges of cannulation of lymphatic vessels and the complication from the oil-based contrast agent, this technique is rarely used today. Duplex ultrasound (DUS) cannot image lymphatic vasculature; however, its use is more reserved for evaluation of venous reflux or obstruction with or without associated phlebolymphedema. Suehiro et al. (2013) have used DUS for evaluation of subcutaneous echogenicity and dermal thickness to correlate with ILS clinical staging of LED. Subcutaneous echogenicity can be graded based on presence and pattern of echogenic lines. If no lines are present, it is graded as 1. In grade II, horizontal echogenic lines are seen, whereas in grade III, diffuse increased and blurred echogenic lines are present. Dependent oedema can be differentiated from LED based on presence of anatomical location and echo-free space (EFS). In dependent oedema, echogenicity is seen more on dependent site with difference in medial and lateral aspects of limb; EFS is more pronounced in lower leg particularly lateral aspect, whereas in LED, there is no difference in medial and lateral aspects with uniform increased in EFS on all sides. Lipoedema shows normal dermal thickness and echogenicity contrary to LED. Lymphoscintigraphy, introduced in 1953 by Sherman and Ter-Pugossian, constitutes the functional evaluation of the lymphatic system and organs with radioisotopes. It is based on one of the essential functions of the lymphatic system, i.e. to transport large molecules from the interstitial space back to the vascular compartment. It is considered as the gold standard diagnostic method for investigation of lymphatic transport. Lymphoscintigraphy has by large replaced the now obsolete radiological method of lymphangiography, which is an invasive modality. Direct lymphangiography involved identification of the lymphatics initially by injection of a dye – Prussian blue followed by cannulation of the lymphatics by a small skin incision. Oil-based contrast medium – iodized lipiodol – is slowly then injected through the cannula for many hours under a graded pressure to depict lymphatic collectors and regional draining lymph nodes. The method is very tedious to perform and carries the risk of damage to the lymph vessels inducing fibrosis and worsening the LED further. Radionuclide lymphoscintigraphy, considered as gold standard modality to visualize lymphatic system, has replaced the invasive radiological lymphangiography. When a large molecule labelled with a radionuclide is injected into the interstitial space, its transport through the lymphatic system can be followed using an external gamma camera to detect the radioactivity emitted from the body. This delineates the path of lymph flow from the site of injection giving a picture of lymphatic function and pathways. It enhances the understanding of pathophysiology of LED and its response to treatment. However, it does not elucidate the degree of fine anatomical detail. Radiotracers: Two main types of preparations are used which are labelled with a radionuclide, commonly 99m-technetium (Tc). The size of these macromolecules ranges from 100 to 1000 nm. The molecules which have been used include Tc99m-human serum albumin (HSA), Tc99m-dextran and Tc99m-human immunoglobulin (HIG). Macromolecules are cleared faster than colloids and produce better images of the lymph vessels. However, they are not trapped in the node like a colloid. Thus, they do not produce good images of nodes. There is also a risk that smaller molecules will enter the blood capillaries. These are cleared more slowly from the injection site and therefore do not show the lymphatic vessels clearly. They are, however, trapped more effectively in the lymph nodes and make them more visible on the scan. Commonly used colloid radiopharmaceuticals include Tc99m-antimony sulphide, Tc99m-sulphur colloid and Tc99m-albumin colloid (nanocolloid). Lymphatic vessels are better visualized with macromolecules, whereas lymph nodes are seen more clearly with colloids. The optimal particle size for lymphoscintigraphy is 50–70 nm. Particles larger than 100 nm may be trapped in the interstitial space for a prolonged period. Particles smaller than a few nanometre will escape into the vascular system. Thus, the appearance of the lymphoscintigram will be dependent upon the radiopharmaceutical used and the particle size. Various colloids available are described in Table 2.11.1. Source: Lee BB, Bergan JJ. New clinical and laboratory staging systems to improve management of chronic lymphedema. Lymphology 22;38(3):122-9. 99 mTc-nanocolloid has a particle size of <80 nm (95% of particles) and is cleared rapidly from the injection site. It has also been argued that this is more “physiological” than some of the other colloids, as it is similar to the normal gel-like nature of the subcutaneous interstitial space. The rate of uptake of the radiotracer depends on the volume injected and the amount of concentration of the tracer. These may affect the hydrostatic and oncotic pressures of the local tissue at the site of injection. The usual volume recommended for a subcutaneous injection is 0.2 mL. Fat attenuation of the gamma photons can lead to poor visualization of deep lymphatics, which might falsely get projected as lymphatic dysfunction. It is important that for the duration of the study, the radiolabel remains attached to the macromolecule or colloid. With more stable compounds, once it has travelled through the peripheral lymphatic system, the tracer is carried into the venous system via the normal anatomical connections (e.g. the thoracic duct) and then trapped in the liver subsequently. Thus, normally liver is seen in delayed images. Also, urinary bladder is visualized in delayed images, as radiolabel is excreted in urine. Lymphoscintigraphy can be broadly classified into two major techniques – limb lymphoscintigraphy and sentinel node biopsy. It is utilized to diagnose LED in the upper and lower limbs. The main objective of the lymphoscintigraphy is to establish whether there is a lymphatic dysfunction in the limb, if present, at what level the abnormality lies and to decide on the management options. Various routes of injection have been used to visualize different systems of lymphatic system (Fig. 2.11.1). Single-site or multiple-site injections can be given, in which case, the results of the scan have to be interpreted carefully as different lymphatic pathways may be taken by the tracer, e.g. the drainage of first interdigital space in foot is via superficial system, whereas the fourth interdigital space drains via deeper system. Subcutaneous route is considered to be the optimal way for administration for LSG as the fluid accumulation occurring in the interstitium is in the subcutaneous plane. It is, however, suggested that intradermal injection, which raises a papule in the skin, results in an increase in local interstitial pressure, which facilitates entry into the initial lymphatics. There is also a high concentration of lymphatic vessels in the dermis, giving a large surface area for the uptake of tracer. In subcutaneous injections, the pressure increase is less, and the diffusion distance to the nearest lymphatic may be greater, thus leading to a slower uptake. Though subcutaneous route is considered optimal, intradermal route is preferred as it raises the local interstitial pressure, thus facilitating the entry of radiopharmaceutical into the initial lymphatics. It can be demonstrated by the subfascial injection of radiotracers, which include the following routes: A two-compartment lymphoscintigraphy with simultaneous superficial and deep routes of injection can also be performed (Fig. 2.11.1). Patient preparation: No specific patient preparation is needed. On clinical examination, no active infection or changes of cellulitis should be seen on the limb. If present, the procedure should be done at a later date under antibiotic cover. Injection of the radiopharmaceutical: After the injection of radiotracer, maximum up to 1 mCi, with the desired route of injection (most commonly used route is intradermal) in the webspaces of the hands or feet of both the limbs, sweep images are acquired under the gamma camera. Immediate, postexercise and delayed images are acquired. Postinjection, immediate sweep images delineate the lymphatic channel. The number of channels, prominence and tortuosity of the channels can be observed in these images. When a single injection of the radiopharmaceutical is given intradermally in the first webspace, a single channel along the medial side of the limb is seen draining into the nodes. It is well known that exercise accelerates lymphatic drainage by the extrinsic compression of the valved lymphatic vessels contracting skeletal muscles. Thus, the amount of exercise carried out during the course of imaging can affect both qualitative and quantitative results. A number of protocols have been reported in the literature. The term “stress lymphoscintigraphy” is sometimes used in the literature to describe the technique of examining the response of lymphatic flow to an intervention, e.g. exercise. Thirty minutes postinjection or after acquisition of immediate images, if no tracer activity is seen in the groin or axilla, the patient should mobilize or stress their limbs. Lower extremity stress includes walking, bicycle exercise, flapping of feet or limb massage. Upper limb stress includes hand grip or massage and repetitive squeezing of a rubber ball. Delayed imaging is normally done 3 h after injection, normally in vast majority of cases. In some limbs where the nodes are not immediately visualized, delayed images even up to 24 h have to be acquired to check for the visualization of nodes, as the transport and flow of lymph might occur slowly due to the oedema. Like any other nuclear medicine procedure, lymphoscintigraphy reflects the underlying physiology as well as pathophysiology. When a colloid or a macromolecule is injected in the limb, it is treated as the protein molecules in the lymph and is drained to the nodes. Eventually the tracer enters the systemic circulation through the thoracic duct. Various visual grading systems were proposed for lymphoscintigraphy, most popular among them being the one in 2005 by Lee and Bergan. It required the presence of two or more positives of the multiple parameters such as lymph nodal uptake, dermal backflow, collateral lymphatics, main lymphatics and clearance of radioisotope from injection site for each grade. This turned out to be very cumbersome, time-consuming and confusing and was not standardized. So we devised our own institutional system of grading from the vast experience of lymphoscintigraphy over the years. Staging of LED: LED is divided into four stages or grades (Table 2.11.2) on lymphoscintigraphy based on the flow of the tracer and visualization of the nodes. This image-based grading system has been standardized at our institution. Source: Shelley S, Manokaran G, Indirani M, Gokhale S, Anirudhan N. Lymphoscintigraphy as a diagnostic tool in patients with lymphedema of filarial origin—an Indian study. Lymphology 39(2):69-75. The findings on a lymphoscintigram parallel the changes occurring in the limb (Fig. 2.11.3) during development of LED. Owing to the overproduction of lymph (high output) or the inability of the lymphatics to handle the normal lymph flow (low output), the channels become prominent, dilated and tortuous and multiple collaterals are formed (grade I) to compensate for the dysfunction. Under normal conditions, the superficial and deep systems are interdependent such that the deep lymphatic system participates in lymph transport from the skin during lymphatic obstruction. The rate of drainage is different in both systems, the deeper system being slower as compared with the superficial system. When the formation of collaterals does not compensate for the lymphatic dysfunction, the deeper system takes the charge and the lymph flow gets rerouted to deep lymphatics. In spite of injecting the tracer into the superficial lymphatics which are overwhelmed, the deep system – nodes and lymphatics are visualized (grade II). The stasis of protein-rich fluid in the interstitium initiates an inflammatory reaction with increase in macrophage activity, resulting in destruction of elastic fibres and production of fibroelastic tissue. At a later stage, the valves guarding the unidirectional flow of the lymph become incompetent due to the fibrosis, leading to reflux of lymph into the dermal plexus, which can be seen as the dermal backflow (grade III). Finally, fibroblasts migrate into the interstitium and deposit collagen, leading to fibrosis of lymphatic vessels, which results in absent flow through the lymphatic system altogether. This corresponds to no flow of tracer in the limb and nonvisualization of nodes in lymphoscintigraphy (grade IV). Though the visual analysis provides ample functional information about LED, at time the intricate abnormalities at the start of the events are missed. In these scenarios, the quantitative analysis often helps. Quantification can be done by assessment of the rate of clearance of tracer from the injection site with a dynamic acquisition of lymphoscintigraphy postinjection. The nodes (inguinal/axillary) are kept in the field of view, and the time taken to visualize the nodes postinjection is measured. Another method of quantifying is the nodal uptake percentage with appropriate regions of interest (ROIs) on the nodes and injection sites in the delayed sweep images. A composite formula incorporating both can also be undertaken but requires further studies for standardization. Multiple studies have been performed to assess the quantification on lymphoscintigraphy. Nevertheless, no particular technique or method of quantification has been standardized. Recent studies conducted by Kim, Paul et al. in 201 postmastectomy patients showed a promising role of quantitative lymphoscintigraphic parameters such as ratio of radioactivity of affected to normal axilla (RRA) in predicting detection of early pathophysiological changes predisposing to LED. This can lead to treatment of patients even before full-fledge development of LED and revolutionize treatment of iatrogenic LED, because once LED develops, it cannot be cured. Lymphatic system hailed as the “third circulation” acts as a bridge between the circulatory and immune system. It is composed of lymph, lymphatic conduits, primary (bone marrow and thymus) and secondary (spleen and nodes) lymphatic organs and tissues (tonsils, adenoids and Peyer’s patch) (Fig. 2.11.4). CNS lacks lymphatics, where its function is taken over by CSF. The basic unit of lymphatic system is the lymphatic capillary which starts as a blind sac made of overlapping endothelial cells resting on a thin and porous basement membrane and is anchored to the connective tissue with filaments (Fig. 2.11.5). The lymphatic capillaries are larger than the blood capillaries and are approximately 50 µm in diameter. The capillaries join to form precollecting vessels followed by collecting vessels in the deeper cutaneous layers, with one-way valves, which prevent backflow of lymph. The intervalvular space is called lymphagion, the functional unit of collecting vessels. The afferent collecting vessels enter the lymph nodes through the hilum. After filtration by the nodal macrophages, the lymph flows out of the nodes through the efferent collecting lymphatic vessels. These vessels then coalesce to form five lymphatic trunks. There are four paired and one unpaired lymphatic trunks, namely, jugular, subclavian, broncho mediastinal and lumbar. The unpaired intestinal trunk drains chyle from lacteals of the small intestine. Intersection of lumbar and intestinal trunks forms a saccular dilatation at the level of first and second lumbar vertebra called cisterna chyli. Cisterna chyli continues superiorly as thoracic duct at the level of D12. Thoracic duct enters the posterior mediastinum through aortic hiatus and crosses to left at T4–6 vertebral level. It ascends into neck anterior to anterior scalene muscle and phrenic nerve. Lymph from the whole left and right lower halves of the body drains to the thoracic duct, right upper half of the body drains to right lymphatic duct. The ducts drain into the left and right subclavian veins, respectively. The extremities have interdependent superficial and deep (subfacial) systems of lymphatic drainage, which are interconnected by perforating vessels. The usually slower deep system takes over the lymphatic transport when superficial system fails (Fig. 2.11.6). Capillary exodus of fluid exceeds the capacity of venous absorption by 3 L/day, thereby causing accumulation of fluid and large proteins in the interstitial space. This excess fluid moves into the lymphatic capillaries via the gaps between lymphatic endothelial cells, which lack tight junctions and are furthered by active transendothelial vesicular transport. Presence of hyaluronic acid and glycosaminoglycans (GAGs) imparts a negative pressure to the interstitial space, while the lymphatic capillaries have a positive pressure, hinting that the movement of lymph to initial lymphatics involves an active process, by the combined effort of the siphon pressure due to the collecting vessel smooth muscle contraction and episodic increases in interstitial pressure attained by arterial pulsations and muscle contractions (Fig. 2.11.7). The one-way valves present in the collecting vessel prevents the backflow of lymph. Intrinsic generation of action potentials in smooth muscles actively propulses the lymph forward. Normal lymph flow occurs at the rate of 120 mL/h. Lymphatic contraction is controlled by the adrenergic and sensory nerve endings on the wall. Endothelium-derived nitric oxide, heme proteins and oxygen-free radicals inhibit these pacemaker potentials and cause constriction of lymphatic vessels. Tissue oedema (oedema safety factor), exercise, hydrostatic pressure (standing), mechanical stimulation (massage, pneumatic compression) and warm baths are found to increase the contractility. Beyond hydrostatic pressure of 100 mmHg, the further increase in contractility plateaus. Aetiology of LED can be broadly classified into primary/genetic and secondary/acquired. Primary LED can be subclassified into congenital, precox, tarda according to the age of onset. Filariasis, malignancy and its treatment-associated LED account for the major chunk of secondary LED in a developing nation like ours. Though the ultimate presentation of all LED involves a chain of fibrosis and inflammation, the trigger varies. Hence, it is prudent to simultaneously follow the classification and pathophysiology (Table 2.11.3). Congenital Familial (Milroy’s) Syndromic Sporadic Precox Familial (Meige’s) Sporadic Tarda Filariasis Iatrogenic(surgery, radiotherapy, nodal dissection) Posttraumatic Malignancy Obesity Podoconiosis Aplasia/hypoplasia of lymphatics Lymph conduit deformities Primary valvular insufficiency Proximal, distal or combined lymphatic obstruction Nodal obstruction Secondary valvular insufficiency Source: Szuba A, Rockson SG. Lymphedema: anatomy, physiology and pathogenesis, Vascular Medicine 2(4):321-6. Both the kinds of LED cause lymph stasis and lymphatic hypertension, leading to the water and fat accumulation followed by cyclical interplay of adipose tissue remodelling, adiponectin dysregulation, release of proinflammatory and profibrotic cytokines (TNF-α, IL-6, MCP-1, IL-8, IL 4, IL 13) results in fibrosis, manifested by nonpitting oedema (Fig. 2.11.8). There is initial increased recruitment of macrophages which will cause lymphangiogenesis and collateral development and deroutment to deep system. In late stages, there is macrophage depletion (reduced VEGF-C, nitric oxide synthase expression), which results in impaired lymph angiogenesis and lymphatic drainage. In many patients, LED causes opening of integumentary channels, entry of bacteria to subcuticular plane and activation of host defence mechanisms, paving way for cellulitis. Inability to handle the amount of lymph due to failure of high-output or low-output mechanisms causes lymph stasis in the interstitium causing lymphatic hypertension. This further initiates a vicious cycle (Fig. 2.11.9) of local inflammation with recruitment of inflammatory mediators, resulting in inflammation and eventually fibrosis of the local tissue and the lymphatic channels. The channels become dilated tortuous and form collaterals trying to compensate for the reduced lymph drainage. Majority of the lymphatic drainage occurs through the superficial system in normal conditions and at a faster rate as compared with the deeper lymphatics. When the lymphatic burden increases further, there is rerouting of the lymphatic flow to the deeper system. Fibrosis of the channels and surrounding tissue causes valvular dysfunction, leading to lymphatic reflux into the dermal plexus. Repeated inflammation in the skin ensues, thus causing plaquing, nodularity and hyperpigmentation, thus causing the skin changes seen in LED. In filariasis, due to the filarial infestation and host immune response, humongous swelling of the lower limbs (commonly) can occur, leading to grossly incapacitating elephantiasis. Development of lymphatic system starts in the embryo right from the fifth week of gestation. Many a number of conflicting ideas have been proposed from the animal studies conducted in the past decade, especially in zebra fish and mice. There is more evidence pointing towards the dual origin of lymphatics: (1) venous and (2) nonvenous. Incorporation of paraxial mesoderm into the dorsolateral aspect of cardinal vein, followed by prox 1 expression-induced lymphatic specification and migration along VEGFR3 (vascular endothelial growth factor receptor) gradient to form lymphatic sacs form the basis of venous origin (Fig. 2.11.10). Though transdifferentiation from surrounding mesenchyme is considered as a possibility, the source of nonvenous origin is yet undetermined. Primary LED is due to in utero developmental or genetic defects in development of lymphatic system or lymph nodes. Sporadic (not familial) cases are the most common form of primary LED, Meige’s disease being the most common cause. An identifiable genetic mutation (hereditary LED and hereditary-associated conditions) is estimated to be seen in 30% of patients with primary LED. Primary LED has also been classified according to the timing of presentation as congenital LED (Milroy’s disease) with onset at less than 2 years of age, LED praecox developing around the time of puberty or LED tarda presenting in adults older than 35 years. However, this classification is not much useful as it does not emphasize pathological process. In 2013, Connell et al. (2013) proposed a modified classification pathway and diagnostic algorithm of primary LED based on syndrome association, congenital or late onset, affected body part (systemic or visceral) with the presence of associated features, e.g. dysmorphology, distichiasis, vascular malformations and limb overgrowth. This classification focuses more on detailed combination of genotype/phenotype features for the approach of lymphatic system disorder (Table 2.11.4). Source: Grada AA, Phillips TJ. Lymphedema: pathophysiology and clinical manifestations, Journal of the American Academy of Dermatology 77(6):1009-20. It is a familial congenital predominantly autosomal dominant disorder with mutation of FLT4 gene encoding VEGFR3, resulting in lymphatic hypoplasia and dysfunctional valvular system. The disease usually manifests shortly after birth, within the first year of life as uni/bilateral limb oedema with female preponderance. Most syndromes associated with LED usually have lower limb LED. Familial precox also known as Meige’s disease is the most common cause of primary LED accounting for 65%–80% of cases and occurs mostly around puberty (within 35 years). It is an autosomal dominant disease with variable penetrance and LMPH2 gene mutation. Histologically, it is characterized by hypoplastic pattern. Females are most commonly affected; 70% cases are unilateral. It is the rarest form of primary LED, which has late onset (>35 years) and is usually triggered by trauma/infection. There are hyperplastic tortuous lymphatics causing valvular incompetence. The most important clinical indications of lymphoscintigraphy are primary LED, filarial limb and postlymphadenectomy or radiotherapy status. The primary pathophysiology includes lymphadenodysplasia (lymph node) or lymphangiodysplasia (lymphatic vessel). The lymphoscintigraphic findings are very typical for primary LED with either grade III or grade IV LED (Fig. 2.11.11 and2.11.12). Lymphatic channels or lymph nodes are not visualized. There can be significant lymph nodal gaps (due to absence of nodes). Majority of the cases show lymphatic reflux into the superficial lymphatics termed as dermal backflow. Diagnosis of LED poses a challenge to the physician especially in its early stages. When a patient presents with limb oedema, the first and foremost step is to rule out systemic causes of oedema including cardiac, hepatic and renal failures. If any cause is elicited, the patient should receive the specific management. A detailed clinical history about trauma, systemic symptoms, travel to endemic areas of filariasis, lymphadenectomy and any other surgeries to the limb should be noted. Oedema due to venous insufficiency has to be evaluated with Doppler ultrasound. Lymphatic dysfunction can be diagnosed using lymphoscintigraphy or MRL, which can also determine the lymphatic abnormalities associated with phlebolymphedema and lipolymphedema. Lipedema (oedema due to obesity) is usually a diagnosis by exclusion (Table 2.11.5). Source: Gasparis AP, Kim PS, Dean SM, Khilnani NM, Labropoulos N. Diagnostic approach to lower limb edema, Phlebology 35(9):650-5. In 2013, the International Society of Lymphology revised the Clinical Staging of Lymphedema, which was proposed in 1995 which is mentioned in (Table 2.11.6 and Fig. 2.11.13). Source: International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology. 2013;46(1):1–11. The management of LED is a personalized approach which primarily aims to reduce the morbidity and improve the quality of life of the patient. The treatment should be offered at an earliest detectable point during the evolution of the disease. The management can be conservative, medical or surgical interventions. Patients presenting with unilateral LED may have clinically indolent subclinical LED in the contralateral limb. This group of patients are identified only by lymphoscintigraphy, which facilitates early diagnosis and preventive conservative measures to halt/delay progression to full-blown lymphedema. Staging of LED by lymphoscintigraphy helps in deciding on the management too. Stages 1–2 can be considered for conservative and medical management before surgical intervention. Stage 3 needs surgical intervention, and also the functional mapping would guide the surgeon choosing the different surgical techniques (Table 2.11.7). Physical therapy known as decongestive physiotherapy is the mainstay of managing early stages of LED, which consists of three main goals – to improve lymphatic function, soften fibrosclerotic tissues and reduce the risk of opportunistic infections at the affected site. This can be performed in two phases: Initial intensive phase consists of manual lymphatic drainage using compression bandages and manual lymphatic drainage massages for 2–4 weeks followed by maintenance phase with proper fitting compression hosiery to maintain the effect produced by intensive phase. Fitted garments with high compression classes (30–40 mmHg) are preferred. The patient should be motivated to exercise and to maintain proper weight control and hygiene. Pneumatic compression with multichamber devices, low-level laser therapy and application of vibration, heat and magnetic field have also been tried. The efficacy of this method is limited in later stages of LED, and it does not prevent progression and complications of the disease. This moreover requires a good patient compliance. Coumarin derivatives (α-benzopyrones) and flavanoids (γ-benzopyrones) are utilized. Benzopyrones reduce the vascular permeability, thus reducing the lymphatic load. Additionally, they increase macrophage activity, resulting in increase in proteolytic activity which enhances fluid clearance and tissue composition. This reduces the protein and extracellular fluid accumulation, increasing the lymphatic contractility and flow. However, coumarin is hepatotoxic which limits its utility in treatment of LED. Combination of physical and medical methods has been attempted, but the hepatotoxic effects of benzopyrones are still unsettling. Antibiotics (broad spectrum) and antifilarials (diethylcarbamazine – DEC, Suramin, Ivermectin) are employed to prevent dermatolymphangioadenitis (DLA) in the affected limb. Broad-spectrum antibiotics should be given for at least 3–7 days followed by low-dose penicillin to decrease the risk of DLA. Multiple pharmacological prospects targeting underlying inflammation are in study. Promising targets include inhibition of transforming growth factor (TGFβ) and T helper 2 type cells which are the driving force behind lymphangiogenesis. Excessive generation of immature lymphatic vessels can be prevented by inhibiting T helper 1 and 17 type cells. Surgical procedure can be either physiological or ablative: This is a microsurgical procedure which involved shunting of lymphatic fluid into the venous system. The distal lymphatic channels/nodes (nodovenous shunt) are anastomosed with adjacent subdermal veins bypassing the proximal lymphatic obstruction. Patients of clinical stages I and II who have received conservative therapy but have shown very minimal improvement are candidates for LVA and those who have a functioning lymphatics and lymph node basin (grades I and II LED elicited by lymphoscintigraphy) are also suitable for this method. End-to-end or end-to-side anastomosis can be performed. Preoperatively the lymphatic channels are identified by indocyanine green (ICG) lymphography or by injection of blue dye (isosulfan blue) intraoperatively. LVA is technically difficult and does not have any long-term outcomes. It is not curative and fibrosis or failure of anastomosis can lead to relapse. Patients with significant dermal backflow and no functioning lymphatics or dysfunctional lymph node basin (grades III and IV on lymphoscintigraphy) are suitable for vascularized lymph node transfer (VLNT). The transferred node acts as a sponge which absorbs the local lymphatic fluid and drains into the vascular system. The node also produces vasculoendothelial growth factor–C (VEGF-C) which promotes lymphangiogenesis by formation of afferent and efferent connections between the transferred node and recipient site. The node to be transferred is usually harvested from the patient’s body either from the right supraclavicular region or from the groin. This can result in secondary lymphatic dysfunction at the donor site. These procedures can be considered in patients with clinical stage II or III LED with excess adipose deposition and fibrosis in the limb. The excess skin and subcutaneous tissues are removed from the affected limb to decrease limb volume and improve functional status. Liposuction is one such method which is minimally invasive and low risk and does not compromise lymphatic dysfunction further. Other methods include Charles procedure and staged subcutaneous excision. Acquired lymphatic dysfunction due to radiotherapy or surgical lymphadenectomy is being treated with nanofibrillar collagen scaffolds (biobridges) supplemented with lymphangiogenesis factor VEGF-C. These biobridges guide the lymphatic regeneration when placed in the field of lymphatic dysfunction. It is the most common cause of LED in India and is endemic in coastal areas where culex mosquitoes harbouring Wuchereria bancrofti deposits the larvae in the human body, which proliferate and block the lymphatic channels. Bancroftian filariasis accounts for 90% of the lymphatic filariasis. Other filarial parasites include Brugia malayi and Brugia timori. The larvae deposited on the skin of the human host by mosquito bite penetrate the skin and enter the lymphatics where they complete their cycle to become adult worms which cause endothelial activation in the lymphatics. This causes lymphatic dysfunction either due to the endothelial damage or due to the mutations which are induced by the endothelial activation. Another crucial mechanism of inflammation in the lymphatics is attributed to the dead worm which is hypothesized to be due to the release of Wolbachia – rickettsia-like organisms which live symbiotically with the filarial worms. When the adult worm dies, there is release of these bacteria and their antigens, which intensifies the inflammatory activation further worsening it. Chronic lymphatic insufficiency due to dilatation and dysfunction of lymphatic drainage results in LED. Moreover, persistent inflammatory reaction leads to repeated attacks of acute dermatolymphangioadenitis (ADLA) and manifests as verrucous, warty, humongous swelling of bilateral lower limbs (elephantiasis). Nodal dissection or excision of nodes in breast cancer or melanoma causes lymphatic injury, activation of dendritic cells and danger-associated molecular patterns (DAMPs) triggering the innate immunity (Fig. 2.11.14). This leads to activation of CD4 cells and release of inflammatory cytokines, causing fibrosis and impaired lymphangiogenesis, lymphatic drainage, finally resulting in LED. Radiotherapy leads to loss of dermal lymphatic vessels and nodal fibrosis. There is usually unilateral chronic limb swelling in iatrogenic cases. Considered as endemic nonfilarial elephantiasis, it occurs endemically in tropical Africa, central America and north west India. Chronic bare feet exposure to irritant silica containing soil causes genetically predetermined inflammatory reaction to the minerals in it, progressing to ascending, bilaterally asymmetric lymphatic obstruction. Diagnosis is by exclusion. LED can be the only morbid manifestation of this hidden disease, and this complication causes long-term suffering, morbidity and also social and economic burden to the individuals. The lymphoscintigraphic patterns seen in lymphatic filariasis include multiple dilated tortuous collateral lymphatic vessels most commonly (Fig. 2.11.15). Deep system is visualized more often. In clinically and pathologically advanced cases, grade III and grade IV LED are also seen. Scrotal involvement is not an uncommon finding (Fig. 2.11.16). Lymphoscintigraphy upgrades the stage of LED, thus influencing the management. Especially in cases of unilateral LED, lymphoscintigraphy is a sensitive tool to detect even subclinical LED in the normal appearing contralateral limb. It thus ensures earlier diagnosis and earlier intervention, thus reducing the morbidity. Lymphoscintigraphy shows immediate visualization of lymphatic channels on injection but no axillary or inguinal nodes to drain even in the delayed images. The lymphatic channels are usually prominent and dilated signifying tracer stasis. Quantitative analysis with rectangular ROIs on both the upper limbs can be done using asymmetry index in patients of carcinoma breast posttreatment. The limb on the operated site will show more tracer stasis than the contralateral limb. This is applicable only for the limbs with no significant dermal backflow (Fig. 2.11.17). Another upcoming indication for lymphoscintigraphy in secondary LED is the therapy response assessment. The acquired lymphatic dysfunction is being treated using nanofibrillar collagen scaffolds (Bio bridges) supplemented with lymphangiogenesis factor VEGF-C. These biobridges guide the lymphatic regeneration when placed in the field of lymphatic dysfunction. However, this is still in investigational stages and needs retrospective studies to establish the clinical value. Other prominent clinical indications include chylothorax (Fig. 2.11.18) and chyloperitoneum where lymphoscintigraphy is utilized to localize the site of abnormal communication with the advent of SPECT-CT (single-photon emission computed tomography/CT). One of the indications under rare circumstances includes differentiating between oedema due to lymphatic dysfunction or liver failure. The sentinel node biopsy (SLNB) is a multidisciplinary procedure based on Morton’s principle which states that the afferent lymphatic channel from a primary tumour drains first to one or more sentinel lymph nodes (SLN) also regarded as the gate keeper in the regional lymphatic basin (Fig. 2.11.19). The migration of metastatic cells from tumour to a node or nodes may be relatively quick or may take months, even years. The technique mimics the natural migration of the cells with migration of known detectable tracers. However, the migration of the tracers to the SLN or SLNs is to be within minutes or hours rather than months or years. This is due to the obvious difference between the sizes of a radiolabeled colloid particle and a tumour cell – less than 0.22 mm (colloid) vs 15–20 mm (tumour cell), or about two orders of magnitude difference.
2.11: Lymphatic ducts and disorders
Introduction
Imaging techniques of lymphatic system
Radiological imaging
Nuclear medicine imaging
Lymphography
Role of duplex ultrasound
Lymphoscintigraphy
Principle of lymphoscintigraphy
Macromolecules
Colloids
Factors affecting lymphoscintigraphy
Size
PARTICLE SIZE (nm)
Agent
Maximum
Mean
Sulphur colloid
350–5000
100–220 (filtered)
Antimony trisulphide
80
3–30
Sulphide nanocolloid
80
10–50
Nanocolloidal albumin
100
5–80
Rhenium sulphide nanocolloid
500
50–200
Tin colloid
800
30–250
Labelled dextran
800
10–400
Hydroxyethyl starch
1000
100–1000
Stannous phytate
1200
200–400
Tilmanocept (lymphoseek)
7
7
Volume and quantity of tracer injected
Obesity
Stability of the radiotracer
Technique
Limb lymphoscintigraphy
Indications of limb lymphoscintigraphy
Route of injection
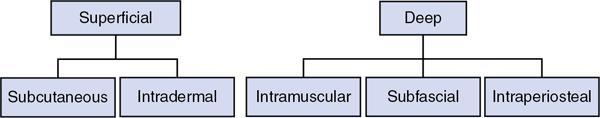
Route of injection for superficial system
Route of injection for deep system
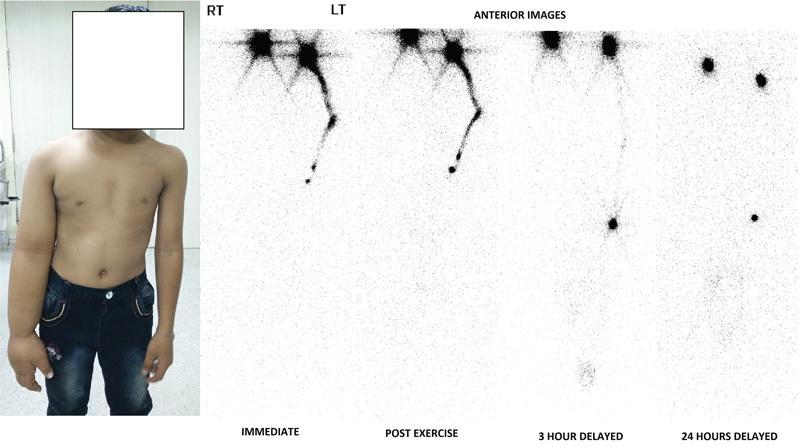
Procedure
Immediate
Postexercise imaging/stress lymphoscintigraphy
Delayed
Interpretation
Normal pattern of lymphoscintigraphy (Fig. 2.11.2)
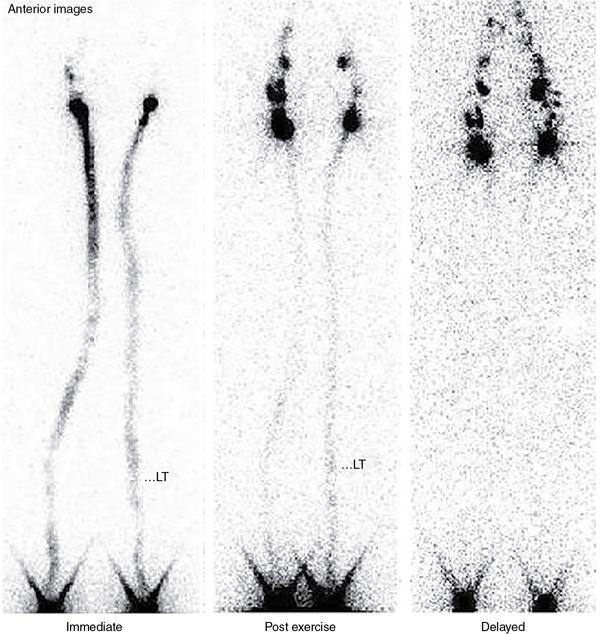
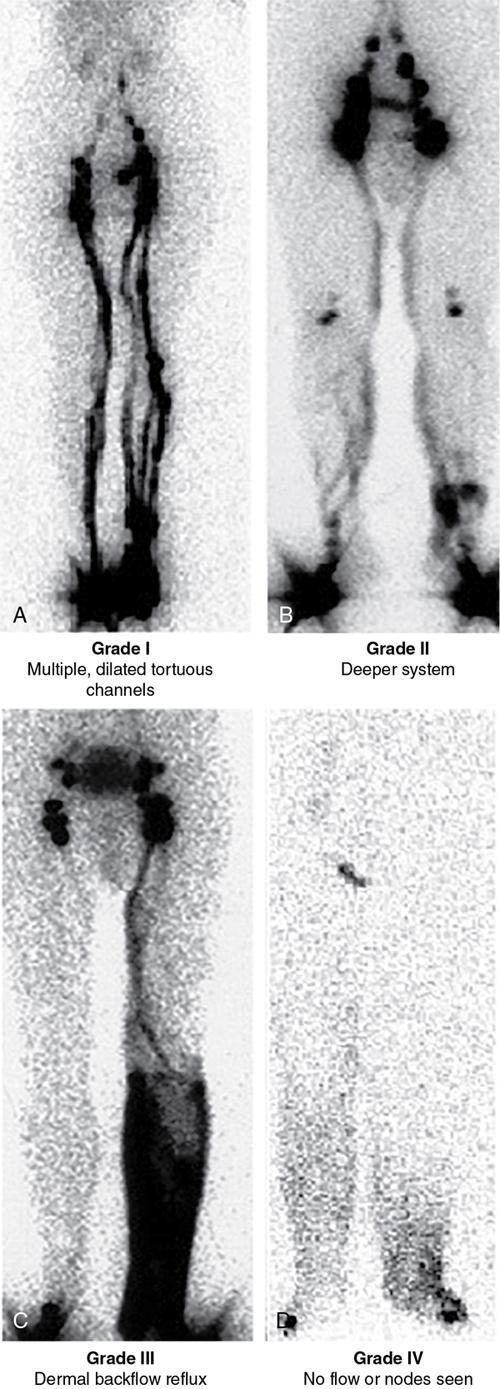
Visual staging of lymphoscintigraphy
Grade I – Multiple or dilated lymphatic channels or delay to visualize lymph nodes in immediate images
Grade II – In addition to grade I findings, flow through deeper lymphatic system (popliteal lymph nodes)
Grade III – Tracer stasis or dermal backflow in the delayed images. Lymph nodes visualized in delayed images
Grade IV – Grade I, II and III with nonvisualization of lymph nodes
Quantitative analysis
Anatomy of lymphatic ducts and various lymph node stations
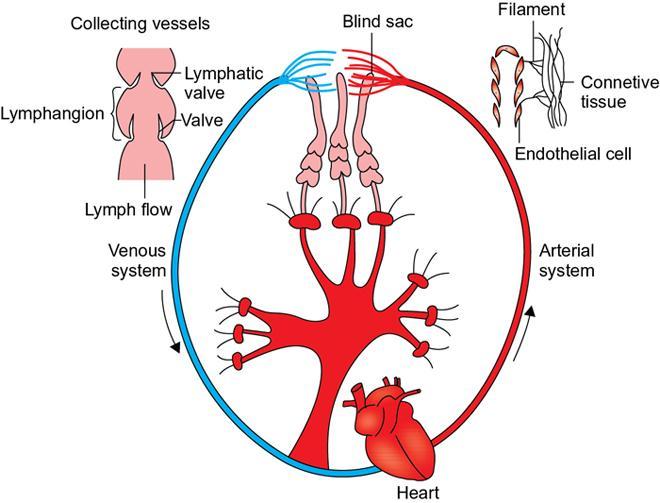
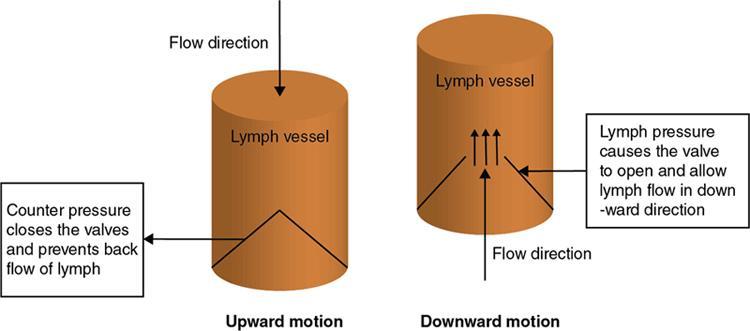
Physiology of lymph flow
Aetiology, classification and general pathophysiology of lymphedema
Classification
Primary Lymphedema
Secondary Lymphedema
Causes
Initiating event
Embryology of lymphatic system
Paediatric generalized lymphatic diseases
Primary lymphedema
Syndrome
Gene
Associated Findings
Oculodentodigital dysplasia
GJA1
Microcephaly, microphthalmia, syndactyly of 4th, 5th fingers
Turner syndrome
45XO, SHOX
Webbed neck, low posterior hairline, lymphedema, primary amenorrhea, aortic coarctation
Choanal atresia syndrome
PTPN14
Bilateral bony choanal atresia
Parks weber syndrome
RASA1
Port wine stain, arteriovenous fistula
Noonan syndrome
PTPN11
Short stature, hypertelorism, nuchal oedema, pigmented nevi, cafe au lait macules, keratosis pilaris atrophicans, keloid formation, coarse light-coloured curly hair, hypogonadism, pulmonary stenosis
SOS1
KRAS
Klippel–Trenauny–Weber syndrome
KTWS
Limb overgrowth, cutaneous angiomas and venous disease
Aagenaes syndrome
LCS1
Obstructive jaundice
Tuberous sclerosis
TSC 1,2
Facial angiofibromas, gingival fibromas, ash-leaf macules, seizures, shagreen patch, dental enamel pits, neuropsychiatric defects
Hennekam lymphangiectasia syndrome
CCEB1, FAT4
Lymphedema (face and lower extremities), intestinal lymphangiectasia, intellectual deficit, facial dysmorphism
Lymphedema – distichiasis syndrome
FOXC2
Secondary set of aberrant eyelashes arising from meibomian glands, ptosis, venous reflux
Yellow nail syndrome
FOXC2
Yellow nails and respiratory tract involvement
Emberger syndrome
GATA2
Lymphedema of lower extremities and genitalia, immune dysfunction, cutaneous warts, deafness
Milroy’s disease
Lymphedema precox
Lymphedema tarda
Syndrome-associated lymphedema (Table 2.11.4)
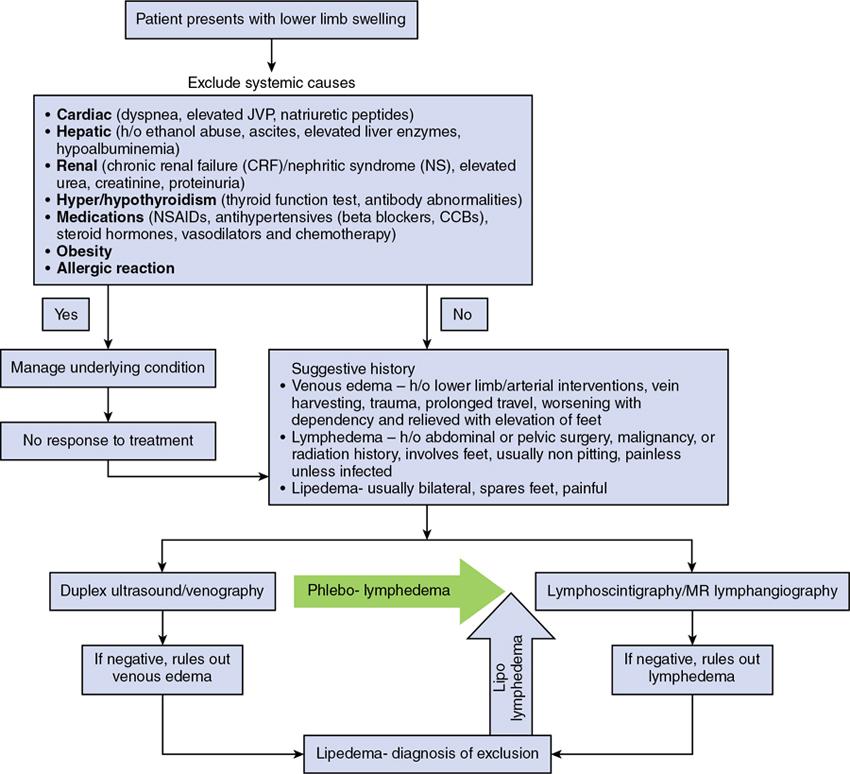
Imaging findings
Primary lymphedema
Generalized lymphadenopathy and approach
Clinical staging of lymphedema
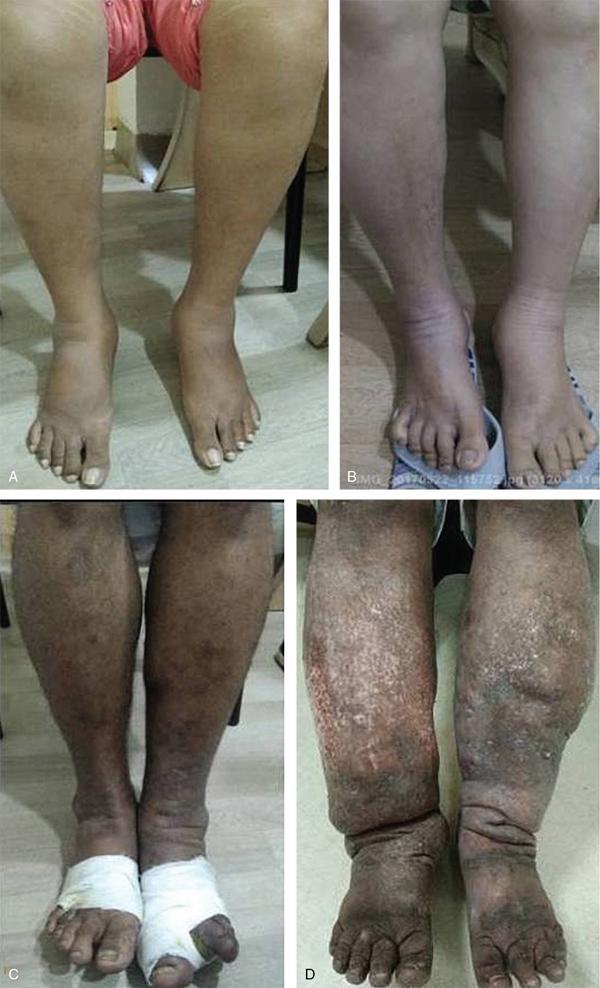
Stage 0 (or Ia): subclinical condition where swelling is not yet evident despite impaired lymph transport, subtle changes in tissue fluid/composition.
Stage I: early accumulation of high protein content fluid with an increase in various proliferating cells. Limb pitting +. LED subsides with limb elevation.
Stage II: Early: Clear limb pit and oedema rarely reduces with limb elevation alone.
Late: limb pitting may or may not present depending on status of accumulation of excess fat and fibrosis.
Stage III (lymphostatic elephantiasis): Trophic skin changes such as acanthosis, further deposition of fat and fibrosis and warty overgrowths. Pitting oedema is usually absent.
Management of lymphedema
Conservative methods
Decongestive lymphatic therapy
Medical and pharmacological management
Benzopyrones
Management of infection
Emerging therapies
Surgical interventions
Physiological procedures
Lymphovenous anastomosis/bypass
Vascularized lymph node transfer
Ablative procedures
Recent advances
Biobridging
Adult lymphatic diseases
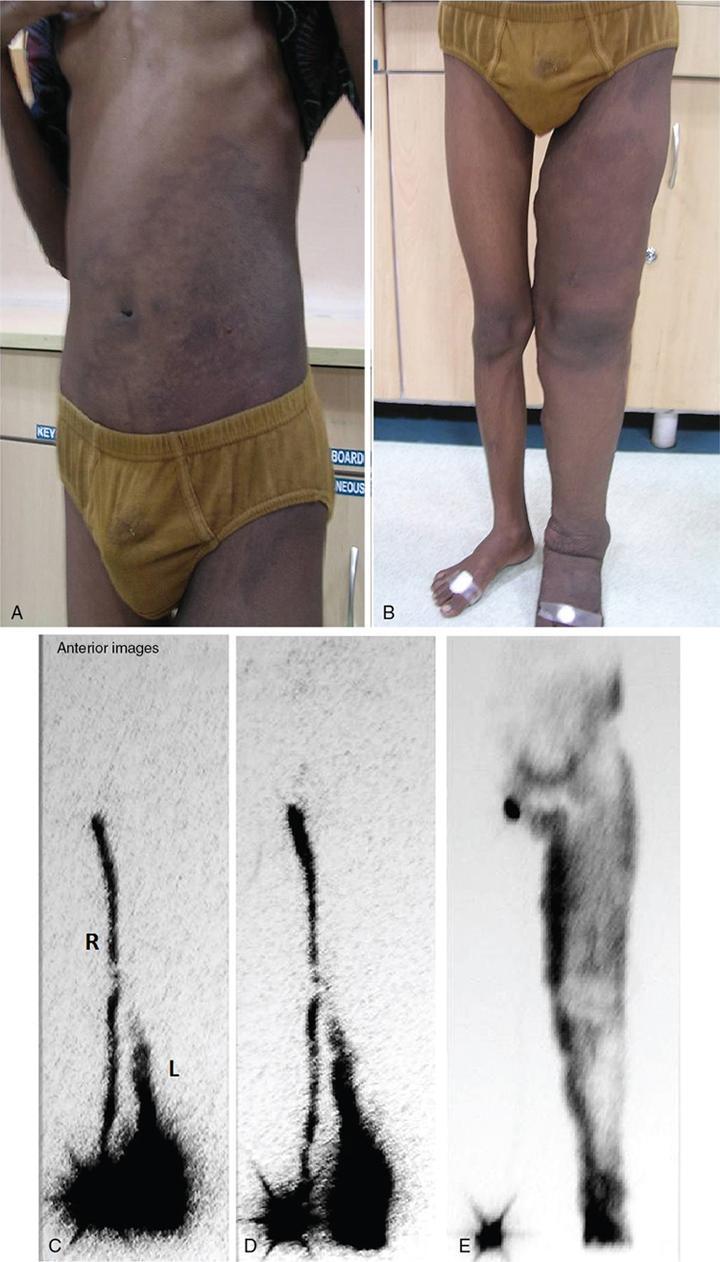
Filariasis
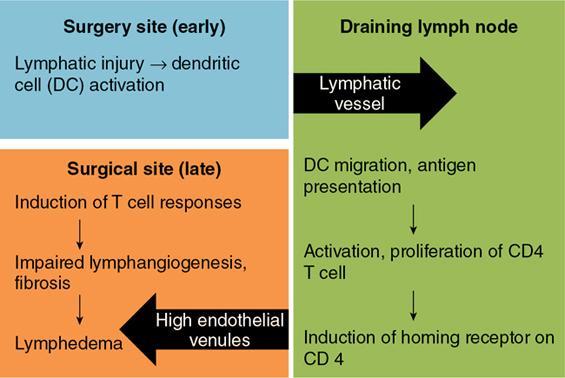
Iatrogenic
Podoconiosis
Imaging findings
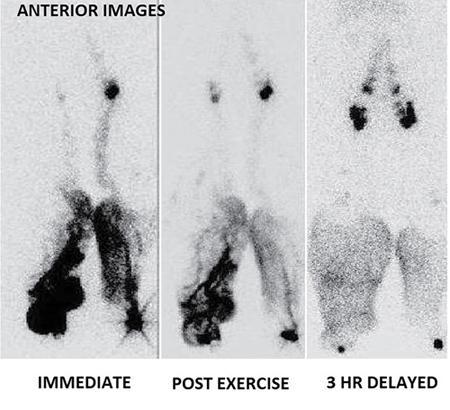
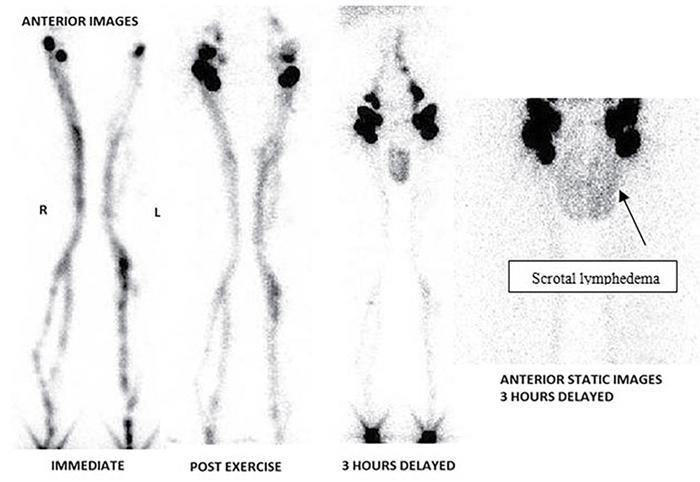
Filarial limb
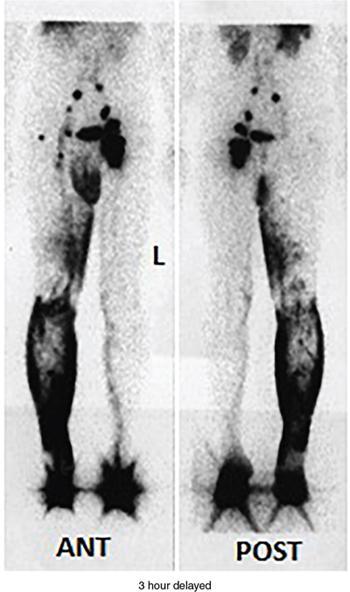
Postlymphadenectomy and radiotherapy
Other indications of lymphoscintigraphy
Lymphatic interventions and lymph node biopsies
Sentinel node biopsy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree